Summary of Key Points
- •
Of key importance is whether a prognostic marker is also a predictive marker for therapeutic benefit.
- •
As predictive biomarkers become integral in the use of targeted therapies to treat lung cancer, multidisciplinary and evidence-based guidelines for molecular testing are needed; the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association of Molecular Pathologists have published a multidisciplinary and evidence-based guideline for molecular testing in lung cancer.
- •
Immunohistochemistry (IHC) is considered to be an easy and inexpensive clinically applicable assay.
- •
The method for detecting mutations needs to take into account the tumor content available, the possibility of detecting all mutations, the timing required for testing, and the urgency to start the patient’s treatment.
- •
Mutation testing should be performed on formalin-fixed paraffin-embedded, frozen, or alcohol-fixed tissue specimens.
- •
The advantage of IHC and fluorescence in situ hybridization is that the evaluation of the protein expression level or genomic aberration can be analyzed more specifically on individual tumor cells.
- •
The choice of the biomarkers to be tested must be based on evidence of their clinical relevance for therapeutic decision.
- •
Epidermal growth factor receptor (EGFR) testing should be done for any mutation located in exons 18 to 21 with over 1% prevalence.
- •
Currently, EGFR mutation is detected by classic molecular tests.
- •
In addition to EGFR, the other approved targetable biomarker used in the treatment of patients with advanced lung cancer is anaplastic lymphoma kinase (ALK) gene rearrangement, which should be done on the same patient population as tested for EGFR mutations.
- •
Aside from EGFR mutations and ALK rearrangements, several other biomarkers have also been tested for their ability to predict lung cancer response to treatment, but none have shown sufficient evidence for current use in clinical practice.
- •
Tests for EGFR mutations, ALK, and ROS gene rearrangements to predict response to EGFR tyrosine kinase inhibitors (TKIs) and ALK/ROS1 TKIs, respectively, are currently the only biomarker tests recommended in clinical practice.
Biomarker research in lung cancer aims to characterize prognostic factors and to determine predictive markers of benefit, usually in terms of response rate or outcome from local treatment (e.g., radiation) or systemic treatment (e.g., chemotherapy, targeted therapy, and immunotherapy). These biomarkers can be used to select the patient groups who will most likely derive differential benefit from the treatments and can help to avoid the toxicities associated with ineffective therapies. It is important to distinguish between prognosis and prediction. Prognostic factors are patient- and tumor-related factors that predict patient outcome (usually survival) and are independent of treatment administered. Predictive factors are clinical, cellular, and molecular markers that predict response of the tumor to treatment (either in terms of tumor shrinkage or a survival benefit from treatment). Therefore prognostic factors define the effects of tumor characteristics on the patient, whereas predictive factors define the effect of treatment on the tumor. Those measures are not always similar, as tumor response may not necessarily translate into greater survival benefit.
Many candidate prognostic biomarkers have been reported to be associated with earlier stages of nonsmall cell lung cancer (NSCLC) in patients who are treated primarily by surgical resection. However, it should be emphasized that not all prognostic classifiers that may predict survival will be associated with the benefit of adjuvant chemotherapy. For this reason, it is important to demonstrate if a prognostic marker is also a predictive marker for therapeutic benefit. In this chapter, we mainly focus on clinical recommendations for the use of molecular testing as predictive biomarkers for response and outcome to systemic therapy, as there has been strong evidence for implementation of routine molecular testing in standard clinical practice. We also discuss the research data on prognostic biomarkers.
Genetic Abnormalities in Lung Cancer
The epidermal growth factor receptor (EGFR) mutation is the first molecular abnormality in lung cancer that has been associated with marked sensitivity to a tyrosine kinase inhibitor (TKI) with specificity for the EGFR. This discovery revolutionized the diagnosis and treatment of lung cancer and established the paradigm for subsequent research to identify oncogenic driver mutations that could represent additional targets for the treatment of lung cancer. Shortly after the discovery of EGFR mutations, gene rearrangement involving the anaplastic lymphoma kinase ( ALK ) was identified as a potent oncogene in NSCLC and has become a predictor of very high rates of response and good outcomes with crizotinib, which inhibits hepatocyte growth factor receptor and ALK. By direct and next-generation high-throughput sequencing, other mutations have subsequently been identified in different histologic types of lung cancers. First in lung adenocarcinoma, Ding et al. reported on a set of 26 genes with significant mutations selected on the basis of statistic models, including known tumor suppressor genes (tumor protein 53 [P53], serine/threonine kinase 11 [STK11], neurofibromatosis 1 [NF1], ataxia telangiectasia mutated [ATM], adenomatous polyposis coli [APC], cyclin-dependent kinase inhibitor [CDKN2A], retinoblastoma 1 [RB1], inhibin beta A [INHBA]); known oncogenes (Kirsten rat sarcoma [KRAS], neuroblastoma RAS viral (v-ras) oncogene homolog [NRAS]); putative oncogenic tyrosine kinase receptors (EGFR, v-erb-b2 avian erythroblastic leukemia viral oncogene homolog [ERBB] 4, fibroblast growth factor receptor 4 [FGFR4], ephrin (EPH) receptor A3 [EPHA3], EPH receptor A5 [EPHA5], neurotrophic tyrosine kinase, receptor, type 1], kinase insert domain receptor [KDR], neurotrophic tyrosine kinase, receptor, type 3 [NTRK3], platelet-derived growth factor receptor, alpha polypeptide [PDGFRA], leukocyte receptor tyrosine kinase [LTK], p21 protein [Cdc42/Rac]-activated kinase 3 [PAK3]); and other genes with undetermined roles (low-density lipoprotein receptor-related protein 1B [LRP1B], protein tyrosine phosphatase, receptor type, D [PTPRD], GNAS complex locus [GNAS], zinc finger, MYND-type containing 10 [ZMYND10/BLU], and solute carrier family 38, member 3 [SLC38A3]). Other studies using DNA and RNA next-generation sequencing (NGS) reported additional potentially actionable oncogene driver mutations, including ERBB2; v-akt murine thymoma viral oncogene homolog 1 (AKT1); met proto-oncogene (MET); lemur tyrosine kinase 2 (LMTK2); catenin (cadherin-associated protein), beta 1, 88 kDa (CTNNB1); neurogenic locus notch homolog protein 2 (NOTCH2); SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4 (SMARCA4); kelch-like epoxycyclohexanone (ECH)-associated protein 1 (KEAP1), AT-rich interactive domain 1A (SWI-like; ARID1A); U2 small nuclear RNA auxiliary factor 1 (U2AF1); and RNA binding motif protein 10 (RBM10), as well as gene fusions, including c-ROS oncogene 1, receptor tyrosine kinase (ROS1), ret proto-oncogene (RET), fibroblast growth factor receptor 2 (FGFR2), AXL receptor tyrosine kinase (AXL), microtubule-associated protein 4 (MAP4/3K3); and platelet-derived growth factor receptor, beta polypeptide (PDGFR1). More recently, putative targetable mutations/amplifications were identified in lung squamous cell carcinoma, including phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PI3KCA); phosphatase and tensin homolog (PTEN); AKT1–3; FGFR1–3; EGFR; ERBB2; v-raf murine sarcoma viral oncogene homolog B (BRAF); NOTCH; RAS; TP53; cyclin-dependent kinase inhibitor 2A (CDK2N2A [p16INK4A])/Rb; KEAP1; cullin 3 (CUL3); nuclear factor, erythroid 2-like 2 (NFE2L2); SRY (sex determining region Y)- box 2 (SOX2); tumor protein p63 (TP63); NOTCH1/2; achaete–scute family bHLH transcription factor 4 (ASCL4); and Forkhead box P1 (FOXP1). Much less data are available in small cell lung cancer (SCLC) because of the rarity of resected specimens. However, gene amplification has been detected with the use of array-comparative genomic hybridization in Janus kinase 2 (JAK2), FGFR1; and SOX2; and cyclin E1 and MYC family members. Gene mutations have also been reported in SCLC in TP53, RB, PTEN, slit homolog 2 (SLIT2), and EPH7, and in genes playing a role in epigenetic gene regulations as CREB-binding protein (CREBBP), E1A-binding protein p300 (EP300), and myeloid/lymphoid or mixed-lineage leukemia (MLL) genes.
Because EGFR mutations and ALK rearrangement have been found to predict therapeutic benefit with their respective targeted drugs, biomarker testing has been implemented and integrated into therapeutic decision making for patients with advanced NSCLC. As predictive biomarkers are becoming integral in the use of targeted therapies to treat patients with lung cancer, there is a need to establish a multidisciplinary and evidence-based guideline for molecular testing. In 2013, the College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association of Molecular Pathologists (AMP) published such guidelines for molecular testing in lung cancer. Following a systematic review of the literature and consensus meetings as well as public consultation, an expert panel developed 37 guideline items addressing 14 subjects and made 15 recommendations, ranging from tissue acquisition and processing to assay interpretations. Several other guidelines for biomarker testing have been published by other organizations, including the National Consensus of the Spanish Society of Medical Oncology, the Spanish Society of Pathology, and the European Society of Medical Oncology. In addition, specific recommendations for EGFR testing have been published in the Canadian National Consensus Statement, and recommendations for ALK testing have been made by the Italian Association of Medical Oncology/Italian Society of Pathology and Cytopathology and other international groups of authors.
Assay Platforms in Molecular Testing
Protein Expression
Immunohistochemistry (IHC) is most commonly used for protein expression assessment in the clinical context. IHC is a process that is easily performed by investigators because of the short time needed to complete testing and low cost, and due to its applicability to formalin-fixed paraffin-embedded (FFPE) rather than fresh frozen tissue. In addition, IHC may help investigators assess protein expression at the cellular level, thus allowing them to evaluate cellular localization (e.g., membranous, nuclear, or cytoplasmic), topography (e.g., tumor or stromal cells), and heterogeneity of expression and is also applicable to very small specimens, including cytologic samples. However, many preanalytic and analytic factors may influence IHC reactions, resulting in potentially variable staining that may affect the interpretation of the results. Therefore optimizing and standardizing the protocols and conditions are required for each marker tested. Interpreting the results is also observer dependent and may vary between observers, thus requiring standardization of protocols and conditions. Lastly, the scores for defining positive or negative IHC results for their prognostic or predictive value of specific biomarkers need to be well defined and validated in multiple independent cohorts/institutions and clinical trial samples. However, despite the mentioned limitations, IHC is considered to be an easy and inexpensive clinically applicable assay, which already is available in most pathology departments.
Gene Mutations
The technologies available for mutation analyses are associated with different sensitivity. Analytic sensitivity is defined as the lowest percentage of tumor cells or tumor cell DNA concentration in which a mutation is detectable with confidence within replicate assays. The standard method for detecting mutations has been direct sequencing by the Sanger method. This method allows the detection of a minimum of 25% of mutated allele frequency from tissue containing 50% cancer cells cellularity, if the mutation is heterozygous and in the absence of gene amplification. However, mutated driver oncogenes, such as EGFR and KRAS , are commonly amplified implying that a lower number of tumor cellularity in the sample may yield 25% mutated alleles. Bidirectional sequencing and confirmation by repeat sequencing on independently amplified polymerase chain reaction (PCR) products should be performed especially on FFPE tissue (see later). The impact of the lower sensitivity of the Sanger sequencing method resulting in a substantial false-negative response rate (approximately 30%) in the detection of EGFR mutations has been documented.
To overcome the generally lower sensitivity of the Sanger sequencing method, various technologies are available that allow mutation detection at significantly higher sensitivity with a tumor cellularity of as low as 1% to 5% or mutated allele. These more sensitive technologies involve a mutated allele-enriching strategy, including the peptide nucleic acid/locked nucleic acid amplification, the coamplification at lower denaturation temperature-PCR, or the enzymatic digestion of wild-type sequences. The US Food and Drug Administration (FDA) has approved two assays for the detection of EGFR mutation analyses in advanced NSCLC: the Scorpion-amplification refractory mutation system (ARMS) and cobas technologies (Roche Molecular Diagnostics, Pleasanton, CA, USA). Several other methods may be used to detect EGFR mutations ( Table 18.1 ). The Scorpion-ARMS technology is available as a commercial kit that allows investigators to test for 29 EGFR mutations and has a sensitivity of at least 5%. The cobas EGFR mutation test is a reverse transcription-PCR-based (RT-PCR) test for the qualitative detection of exon 19 deletion and exon 21 L858R mutations of EGFR in DNA extracted from FFPE tissue and was used by the investigators in the European Randomized Trial of Tarceva versus Chemotherapy (EURTAC) and LUX-Lung 3 trials.
| Method | Tumor DNA Required (%) | Targeted or Screening Method | EGFR Mutations Detected | Detection of Deletions and Insertions |
|---|---|---|---|---|
| Sanger direct sequencing | 25 | Screening | Known and new | Yes |
| Real time/TaqMan PCR | 10 | Targeted | Known only | No |
| High-resolution melting analysis | 5–10 | Screening | Known and new | Yes |
| Cobas | 5–10 | Targeted | Known only | Yes |
| Pyrosequencing | 5–10 | Screening | Known only | Yes |
| SNaPshot PCR | 1–10 | Targeted | Known only | Yes |
| MALDI-TOF MS-based genotyping | 5 | Targeted | Known only | No |
| Cycleave PCR | 5 | Targeted | Known only | Yes |
| Fragment length and RFLP analysis | 5 | Screening/targeted | Known only | Yes |
| Allelic-specific PCR/Scorpion ARMS | 1 | Targeted | Known only | No |
| MassARRAY | 1 | Targeted | Known only | Yes |
| PNA–LNA PCR clamp | 1 | Targeted | Known only | No |
| Denaturing HPLC | 1 | Screening | Known and new | Yes |
| Massively parallel/NGS | 0.1 | Screening | Known and new | Yes |
| Digital droplet PCR | 0.01 | Targeted | Known only | Yes |
Several other available assays are based on different technologies. Their sensitivities vary, and only certain techniques have the ability to detect new mutations and/or insertions and deletions. In contrast to the Sanger method that lacks sensitivity for samples with fewer tumor cells, the more sensitive methods might give rise to false-positive results and a lower specificity. Therefore it is crucial that the appropriate positive and negative controls always be included in the assay. Of note, the Sanger method detects any mutation including previously unidentified mutations in the sequenced exons, but other assays are designed for specific mutation testing, as in the case of the digital droplet PCR that has a very high sensitivity. Another option could be a two-step procedure, starting with a highly sensitive detection of the presence of a mutation and the subsequent characterization of the mutation. When finding a mutation that has not or has rarely been reported, the results should not be considered as errors until the replicate test confirms or denies it. However, testing all mutations can require more time and might not be suitable for the clinical situation when treatment must be initiated without delay. In such cases, another approach can be used that tests the most common mutations first and then completes the screening of less frequent mutation.
The recent and rapid development of NGS accomplishes massive parallel gene mutation analyses and discovery and requires a small amount of tissue, preferably fresh frozen. This technology uses miniaturized and parallelized platforms for sequencing of millions of short nucleotides (50–400 bases). The different platforms all have in common a technical paradigm of massively parallel sequencing via clonally amplified, spatially separated DNA templates or single DNA molecules in a flow cell. Currently, NGS is used for research purposes rather than to test specific biomarkers. However, with the expansion of the use of molecular biomarkers and the rapidly growing targeted therapies available, using NGS to have a full molecular profiling of the tumor or at least multiplex mutation testing of a panel of biomarkers of interest might become preferable in the future in order to spare time and tissue. In addition, in a recent study, even for individual gene mutation analyses, NGS has been shown to have better sensitivity, as it detected all relevant EGFR mutations for prediction of response to EGFR TKIs in 24 tumors, compared with the Sanger method and pyro-sequencing, which resulted in four and two false-negative results, respectively.
Nevertheless, the clinician’s chosen approach needs to take into account the tumor content available, the possibility of detecting all mutations, the timing required for testing, and the urgency to start the patient’s treatment. Usually a laboratory investigator will decide to choose a specific method based on equipment availability and cost and will conduct an assay optimization and standardization exercise and test for assay sensitivity and specificity.
Changes in Gene Structure and Copy Number
Fluorescence in situ hybridization (FISH) is the standard method for assessing changes in gene structure and copy number. Similar to IHC, FISH can be performed on FFPE tissue but requires standardized protocols ( Fig. 18.1 ). Interpreting and reading FISH specimens is observer dependent and requires a dark room and a specific microscope, and the reader needs specific training and expertise in order to achieve reproducible results. Of note is that the tissue structure is not well visualized, and this factor necessitates the preselection of areas to assess in order to discriminate tumor cells from nonmalignant cells. Furthermore, the fluorescence probes are unstable and fade over a short period, which can limit the possibility of revisiting the specimens. Therefore imaging the specimens in a short time frame is important. By using probes against multiple targets labeled with different fluorescent dyes, it is possible to assess multiple markers on the same sections. In addition to detecting the gene copy number, FISH is used to assess structural changes including fusions between genes. The example of ALK fusion is detailed later in this chapter.
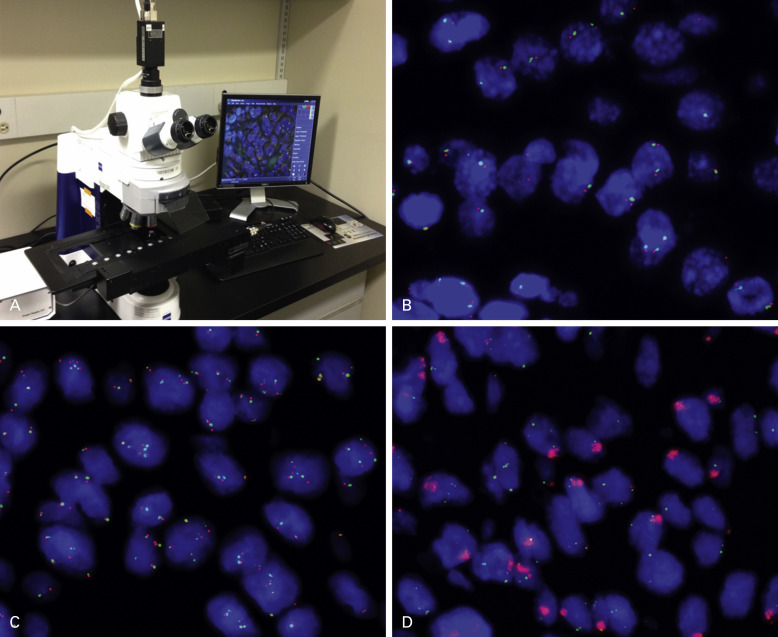
Several alternative techniques to FISH have been developed, including chromogenic in situ hybridization and silver in situ hybridization. These techniques are used primarily in research and give results comparable with FISH; however, they are not commonly used in routine clinical testing in lung cancer. Silver in situ hybridization is now FDA approved for human epidermal growth factor receptor-2 (HER2) determination in breast cancer and is widely used for that indication. Multicolor assays are in development. Finally, gene copy number can be assessed by array-comparative genomic hybridization technique. However, comparative genomic hybridization is used mainly to probe for a large number of markers in exploratory discovery research, rather than to assess specific markers for clinical applications.
Another assay that is used for assessing gene copy number is PCR, which is a very sensitive method that requires specific primers and probes as are used in gene rearrangement in the ALK gene. More recently, computational algorithms have also been developed to derive gene copy number estimate using high coverage NGS data.
Tissue Requirements for Molecular Testing
Preanalytic Factors
Based on expert consensus opinion mutation testing should be performed on FFPE, frozen, or alcohol-fixed tissue specimens. The main advantage of using FFPE tissue is that it is the most commonly used method to process tissue for routine histology. FFPE also allows for a better evaluation of tumor cell content, which is also possible with fresh tissue, but is less convenient and requires cutting and staining of frozen sections adjacent to the section used for DNA extraction. The results of mutation testing with alcohol-fixed tissue specimens are also excellent. This fixation method is often used on cytologic specimens, which are then suitable for mutation testing. DNA isolated from fresh or frozen tissues may yield fragments of 1000 base pairs (bp) and longer. Fixation of tissue in formalin induces crosslinks between DNA, RNA and proteins, and DNA fragmentation that results in DNA fragments of 300 bp or less. Formalin fixation also creates random nucleotide base exchange, resulting in false-positive results. This type of problem mostly occurs with low DNA yield and/or with ultrasensitive assays. Tissue treated with acidic or heavy-metal fixatives, including lead, cobalt, chromium, silver, mercury, and sometimes even uranium and decalcifying solutions, may reduce the success rate of mutation testing and should be avoided when alternate FFPE specimens are available. In molecular biology, heavy-metal fixatives inhibit the DNA polymerases used in PCR testing. Acidic solutions, including the decalcifying solutions that are used to process samples obtained from bone metastasis, can induce a high rate of DNA fragmentation. For these types of specimens that are obtained specifically for molecular testing, nonacidic methods of decalcification, such as nonacidic chelating decalcifying solutions, should be used in the sample-processing step.
IHC and FISH should be performed on FFPE tissue, ideally on cut sections that have been stored for fewer than 6 weeks, to avoid the oxidation process that occurs over time. Whatever the method used, standardizing the fixation procedure and storage conditions is required. The fixation should be performed within hours after the sample has been obtained. Fixation duration should be controlled and not exceed 12 hours for small biopsy specimens and 18 hours to 24 hours for resected specimens.
Data have shown that molecular testing (i.e., mutation testing or FISH) can be performed on liquid biopsies (circulating DNA extracted from plasma or circulating tumor cells). These assays are still experimental and need to be reproduced and standardized before any clinical application.
Sample Processing and Analysis
Tumor tissue is heterogeneously composed of a mixture of tumor cells and host cells. Host cells include inflammatory cells and vascular endothelial and stromal fibroblasts and their abundance is highly variable but may have a substantial impact on the sensitivity of mutation testing ( Fig. 18.2 ). The proportion of tumor cells in the specimen, as compared with normal tissue and inflammatory cells, may affect the result of the mutation analyses, mainly with less sensitive methods for mutation detection, as a low copy number of the DNA template generates artifacts in the results. To avoid false-negative results, specimens with a minimum proportion of tumor cells ideally should be selected for mutation analyses.
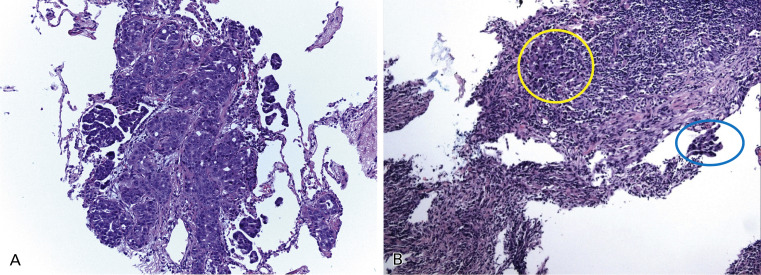
Routinely, DNA is extracted from five to ten scratched unstained sections, depending on the size of tissue sample. However, in some cases, a very low amount of DNA is obtained. To overcome the low amount of DNA extracted from small tissue specimens, different techniques can be used. Whole-genome amplification has been developed and is used in research; however, this technique has not been implemented in clinical testing yet, as it may introduce bias. Performing the assay in duplicate, and ideally in triplicate, may ensure the accurate interpretation of the results, but these methods may not be practical in a clinical laboratory because of the lack of tissue and the time and labor needed to duplicate (or triplicate) testing. Different methods of tissue enrichment can be used for tissue with heterogeneity in areas with tumor cells, including gross macrodissection, coring areas with tumor cells out of an FFPE block, microdissection from the glass, flow sorting, or laser capture microdissection (LCM). Macrodissection is used for clinical testing, but LCM is not routinely used because it is labor intensive and because the effects of a laser on mutation testing are unknown and must be evaluated. In addition, even if LCM produces a very pure sample of tumor cells, it also provides a very low DNA yield.
The advantage of IHC and FISH is that the evaluation of the protein expression level or genomic aberration can be analyzed more specifically on individual tumor cells. In IHC and FISH, cells are analyzed individually. Therefore the tumor cell’s content is less crucial than for mutation testing. However, focusing the analysis on tumor-rich areas is important in FISH. Thus a corresponding hematoxylin and eosin (H&E)-stained section should be used to select areas for analysis. When using IHC, a larger sample size provides a better evaluation of the tumor heterogeneity and percentage of cells expressing the biomarker. However, obtaining larger samples is not easily controlled, as the sample size depends on the type of specimen that can be obtained from the tumor.
Sample Availability and Prioritization of Biomarkers for Testing
Three types of tissue samples can be used for molecular testing. A first approach is used for patients who originally presented with early-stage disease and had subsequent recurrence, a case in which large amounts of the initially resected archival primary tumor should be available for testing. A second approach is used for patients who presented with advanced-stage disease, for whom limited tissue in the form of bronchial, core-needle aspiration biopsy samples of the primary or metastatic tumor, or pleural effusion specimens are suitable for testing. In some instances, if the archival biopsy materials are no longer available or have been exhausted, a new biopsy for molecular testing purposes will be necessary. In all instances, histologic assessment of a freshly cut H&E-stained section of the tissue block should be performed, as part of the preanalytic quality control of the sample. However, in the case of repeat biopsy for testing purposes, there should be a clear indication to the pathologist about the purpose of the biopsy, such that unnecessary ancillary diagnostic IHC studies can be avoided to maximize samples for molecular testing.
Although biopsy tissue samples from patients with advanced-stage disease may be very limited, the tested biomarkers should be prioritized on the basis of their clinical relevance, and the methods used to test them should have a fast turnaround time for therapeutic decisions. In the initial diagnostic workup of biopsy materials, the biomarkers should be rationally and judiciously selected. Because each repeat facing of paraffin blocks results in tissue loss, cutting additional slides for molecular testing, when initially cutting the slides for histopathologic diagnosis, will help if further testing is necessary. However, this option is not always practical as it can increase the laboratory space needed for storing unstained sections, and more importantly, unstained sections stored at room air are no longer optimal for IHC or molecular studies beyond a few weeks or months. The more practical approach is to order and perform all necessary biomarker testing simultaneously or use multiplex techniques. A third approach that is gaining more acceptance is to perform reflex testing, which is automatically initiated by the pathologist at the time of initial diagnosis. This approach provides the most rapid turnaround time and greatest saving of tissue for future additional studies that may be required for new biomarkers or participation in clinical trials.
Although mutation testing is ideally reported in histology samples, in many cases for patients with advanced disease, the only diagnostic material is based on fine-needle aspiration or cytologic samples. Despite the fact that some molecular/protein analyses can be performed on cytology smear specimens, mutation testing has been better performed on cell blocks prepared from these cells. Therefore cell block preparation is recommended in processing cytologic specimens.
The choice of the biomarkers to be tested must be based on evidence of their clinical relevance for therapeutic decision. To obtain consistent and dependable results, molecular testing should be performed in laboratories that are certified by regional or national regulatory bodies and by well-trained personnel using well-maintained equipment. When determining the methodologic and technical strategy for molecular testing, the main concerns include the sensitivity and specificity of the test, the amount of materials required for successful testing, equipment availability, turnaround time, and cost of the test.
Currently Recommended Predictive Biomarkers in Lung Cancer
EGFR Mutations for EGFR TKI Therapy
The EGFR gene is located on chromosome 7. EGFR TK domain mutations are more frequent in East Asian (40% to 50%) than in white (10% to 20%) patients. EGFR mutations are also found more often in never-smokers than in smokers, and in more women than in men. The mutations are mostly found in adenocarcinomas (around 50% in Asian patients and 25% in non-Asian patients), including adenosquamous carcinomas, but they are uncommon in squamous cell carcinomas (5%; Tables 18.2 and 18.3 ).
| EGFR+ | ||||
|---|---|---|---|---|
| No. of Studies | No. of Patients | No. of Patients | Prevalence (%) | |
| Asian/Pacific Islander | 31 | 3452 | 1547 | 45 |
| White | 10 | 3534 | 853 | 24 |
| Black | 3 | 97 | 19 | 29 |
| Hispanic | 4 | 372 | 65 | 17 |
| Asian/Indian | 1 | 220 | 114 | 52 |
| Asian Patients | Non-Asian Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of Studies | No. of Patients | No. of EGFR + | Prevalence of Mutation (%) | No. of Studies | No. of Patients | No. of EGFR + | Prevalence of Mutation (%) | |
| Gender | ||||||||
| Women | 27 | 1760 | 1027 | 58 | 19 | 3098 | 859 | 28 |
| Men | 26 | 1418 | 456 | 32 | 19 | 2165 | 397 | 18 |
| Smoking Status | ||||||||
| Never | 22 | 1442 | 843 | 58 | 18 | 1471 | 666 | 45 |
| Ever | 22 | 1032 | 265 | 26 | 18 | 3723 | 569 | 15 |
| Histology | ||||||||
| Adenocarcinoma | 25 | 2534 | 1278 | 50 | 19 | 5184 | 1266 | 24 |
| Squamous cell | 8 | 168 | 8 | 5 | 9 | 110 | 6 | 5 |
| Adenosquamous | 2 | 6 | 4 | 67 | 2 | 8 | 1 | 13 |
| Large cell | 4 | 15 | 1 | 7 | 6 | 39 | 2 | 5 |
In patients with advanced NSCLCs that harbor an EGFR activating mutation, the response rate to EGFR TKIs is 68% and the progression-free survival is 12 months, whereas in patients with unselected advanced NSCLC, the response rate and progression-free survival are 8% to 9% and 2.2 months to 3.0 months, respectively. The discovery of this difference prompted investigators to conduct studies comparing chemotherapy with EGFR TKIs in NSCLC patients with an activating mutation. The Iressa Pan-Asia Study (IPASS) was the first randomized trial in which results showed an advantage for EGFR TKIs as compared with chemotherapy for first-line treatment of stage IIIB/IV disease in never-smoker East Asian patients with a tumor harboring an EGFR mutation (hazard ratio for progression-free survival, 0.48; p < 0.001). Subsequently, in several other randomized trials, results showed the superiority of treatment with EGFR TKIs for patients with EGFR -mutated NSCLC tumors ( Table 18.4 ).
| Outcomes: EGFR TKIs/Chemotherapy | ||||||
|---|---|---|---|---|---|---|
| Study | Ethnicity | No. of Patients With EGFR Mutation | EGFR TKI | Chemotherapy | Response Rate (%) | Progression-Free Survival (months) |
| IPASS | Asian | 261 | Gefitinib ( n = 132) | Carboplatin/paclitaxel ( n = 129) | 71/47 | 9.5/6.3 HR = 0.48; p < 0.0001 |
| WJTOG3405 | Asian | 117 | Gefitinib ( n = 58) | Cisplatin/docetaxel ( n = 59) | 62/32 | 9.2/6.3 HR = 0.49; p = 0.0001 |
| NEJ002 | Asian | 228 | Gefitinib ( n = 114) | Carboplatin/paclitaxel ( n = 114) | 74/31 | 10.8/5.4 HR = 0.30; p = 0.001 |
| OPTIMAL | Asian | 154 | Erlotinib ( n = 82) | Carboplatin/gemcitabine ( n = 72) | 83/36 | 13.1/4.6 HR = 0.37; p = 0.0001 |
| EURTAC | White | 173 | Erlotinib ( n = 86) | Platinum doublets ( n = 87) | 71/47 | 9.5/5.2 HR = 0.37; p = 0.0001 |
| Ensure PMID: 26105600 | Asian | 217 | Erlotinib ( n = 110) | Cisplatin/gemcitabine( n = 107) | 63/34 | 11.0/5.5 HR = 0.34; (0.22–0.51) |
| LUX-Lung 3 | Any | 345 | Afatinib ( n = 230) | Cisplatin/pemetrexed ( n = 115) | 56/23 | 11.1/6.9 HR = 0.58; p = 0.001 |
| LUX-Lung 6 PMID: 24439929 | Asian | 364 | Afatinib ( n = 242) | Cisplatin/gemcitabine( n = 122) | 67/23 | 11.0/5.6 HR = 0.28; (0.20–0.39) |
EGFR Mutations to Be Tested
Ninety percent of the activating somatic EGFR mutations are short in-frame deletions in exon 19 (most frequently, delE746-A750) and a point mutation in exon 21 (L858R; see Fig. 18.1 ). These two mutations have been largely associated with sensitivity to EGFR TKIs. However, the other 10% of EGFR mutations are of interest for therapeutic decisions as well. The most frequent in-frame deletions in exon 19 are 15-bp and 19-bp deletions, involving three to seven codons centered on the uniformly deleted codons 747 to 749 (Leu–Arg–Glu sequence). However, 9-bp, 12-bp, 24-bp, and 27-bp deletions are also found, as well as 15-bp and 18-bp insertions. Other, less frequent, EGFR activating mutations that have been identified are in exon 18 (E709 and G719X) and in exon 21 (T854 and L861X). All of these mutations result in an EGFR that is constitutively active and may be sensitive to EGFR TKIs.
Both primary and acquired resistance to EGFR TKIs have been reported. After initial response or disease stabilization for several months, a great majority of EGFR mutation-positive tumors eventually become resistant to EGFR TKIs. Another EGFR mutation, the T790M mutation of exon 20, was found to be a resistance mechanism that is acquired secondarily to treatment with EGFR TKIs in almost 50% of cases. Although the T790M mutation is usually acquired, rare cases of NSCLC harbor T790M-mutated cells, with or without an activating mutation, before treatment with EGFR TKIs and, based on preliminary data, the T790M mutation seems to initially confirm resistance to EGFR TKIs. Inherited T790M germline mutations have also been identified in some families with lung cancer.
Some other mutations in exon 20 include S768 and insertions and are associated with initial resistance to EGFR TKIs. As more sensitive mutation assays are available and as more rare mutations are detected, studies are needed to establish the clinical and therapeutic roles of these rare mutations. More recently, a germline point mutation in exon 21 at codon 843 (vv8431) has been reported as the cause of familial lung adenocarcinoma with resistance to EGFR TKIs.
The EGFR in-frame truncated variant (EGFR vIII), resulting from a deletion of exons 2–7 and consequently of a truncation of 801 bp in the extracellular domain, has been rarely reported in NSCLC. The response of a tumor with the EGFR vIII mutation to EGFR TKIs is not known.
In conclusion, EGFR testing should be performed for any mutation located in exons 18–21 with over 1% prevalence.
Assays Used for Testing
Currently, the EGFR mutation is detected by classic molecular tests. Based on expert consensus opinion, as for any other molecular testing, EGFR mutation testing should be performed on FFPE, frozen, or alcohol-fixed tissue specimens. The largest and best available quality tumor specimen should be used, even if the techniques can be adapted, and include tissue enrichment steps for cases where a low amount of DNA is obtained. The different methods for mutation testing have been described earlier in this chapter. The Sanger sequencing method, ideally used by performing bidirectional sequencing and by confirming with additional sequencing, can be used. However, using Sanger sequencing can result in missing approximately 30% of sensitizing mutation because of the relative low sensitivity of this technique. Therefore when using Sanger sequencing, the cellularity limit needs to be higher, at least 20%, and/or it should be used in combination with a more sensitive mutated allele-enriching strategy or both methods (standard and high sensitivity). When choosing the test, the clinician should take into account the clinical situation and the available tumor content. As mentioned earlier, EGFR mutation testing should be performed in a certified laboratory. The method used should test any mutations in exons 18–21 and be highly sensitive to detect any mutation with a prevalence over 1%. Data have shown the feasibility of detecting EGFR mutations in circulating DNA as well as in circulating tumor cells in patients with NSCLC. The sensitivity of EGFR detection in circulating DNA, with the EGFR mutation detected on a tumor considered as the standard, is approximately 70%. Thus circulating DNA could be used for screening in the first-line treatment setting, but further mutation tumor testing would be required on specimens that test negatively. Besides first-line testing, an increased interest has been shown for using liquid biopsies for monitoring molecular abnormalities and, particularly, to detect EGFR T790M mutations at the time of acquired resistance to first-line EGFR TKIs. The third-generation EGFR TKIs have shown very high response rates in patients whose tumor harbors T790M mutation that is resistant to first-line EGFR TKIs. Therefore molecular testing at resistance is required but rebiopsy in a clinical practice may be challenging and the success rate is limited in advanced NSCLC patients. Therefore T790M detection in the circulating DNA has been performed and showed a sensitivity between 40% and 70% according to the technique that was used. Hence, testing the EGFR mutation with blood samples is a promising and attractive alternative, particularly in second and later lines of treatment, because it does not require an invasive procedure (biopsy), but stronger evidence is needed before it can be recommended as first-line testing. Currently, circulating DNA may be used for EGFR testing for first-line treatment in specific clinical settings in which tissue is absent or limited for molecular testing. Circulating DNA may be used to detect EGFR T790M mutations in NSCLC patients with progression or acquired resistance to first-line EGFR TKIs. However, as the sensitivity is modest, any negative results in the blood should be followed by EGFR testing in the tumor sample.
Specific antibodies for the detection of EGFR mutations by IHC have been assessed, and the authors of several studies have consistently reported good sensitivity and specificity for the detection of the exon 21 L858R mutations as well as the EGFR exon 19 15-bp deletions. Unfortunately, the sensitivity is lower for the detection of EGFR exon 19 deletion of other sizes. After validation and standardization, IHC using mutation-specific antibodies could be an option for initial screening for patients with samples with low cellularity that otherwise would not be adequate for mutation testing. Tumors that test negatively by IHC would still need mutation testing. However, this option needs stronger evidence to be recommended for the selection of patients for EGFR TKI therapy.
Proteomic profile using mass spectrometry has also been shown to predict response to EGFR TKIs. From an analysis of serum samples taken from 139 patients with NSCLC before treatment with gefitinib, investigators developed a proteomic signature that was used retrospectively to classify patients according to response, both in the first- and second-line treatment settings. A significant difference in overall survival was found according to the outcome predicted by the proteomic signatures in gefitinib and erlotinib validation cohorts. The proteomic classifier has since been commercialized as VeriStrat (Biodesix, CO, USA). Testing with VeriStrat predicted survival outcome in the Eastern Cooperative Oncology Group 3503 phase II trial of erlotinib as first-line therapy in NSCLC. However, in a retrospective analysis of patients treated in the National Cancer Institute Canada (NCIC) BR-21 study, testing with VeriStrat was found to be prognostic for both overall survival and progression-free survival and was predictive for response but was not able to predict for differential survival benefit for erlotinib. The proteomic classifier, which is unrelated to EGFR mutation status, is currently being validated in several prospective studies. In PROSE (Randomized Proteomic Stratified Phase III Study of Second Line Erlotinib versus Chemotherapy in Patients with Inoperable Non-Small Cell Lung Cancer), investigators prospectively validated the VeriStrat classifier for second-line therapy in patients with advanced NSCLC. Patients were classified as poor or good according to VeriStrat analysis, and the analysis clearly distinguished patients who would benefit from chemotherapy versus EGFR TKI.
Other Potential Biomarkers for EGFR TKI Sensitivity
Other biomarkers have been assessed for their potential association with sensitivity to EGFR TKI, including EGFR gene copy number and EGFR expression.
Tumors that are EGFR positive on FISH (including high polysomy and gene amplification using the Colorado scoring system ) have been found in 22% to 76% of patients with NSCLC and are associated with a 30% rate of response to EGFR TKIs. However, the EGFR gene copy number is not recommended for prediction of response to EGFR TKIs for several reasons. The response rate of 30% among patients with an increased EGFR gene copy number remains much lower than the response rate of 68% in patients with activating EGFR mutations. In addition, there is a strong association between EGFR mutations and gene amplification and the higher response rate with gene amplification is a consequence of the association with EGFR activating mutations. Some studies, including IPASS, have involved both EGFR gene copy number and EGFR mutation analysis. The response rate for patients with tumors harboring the EGFR mutation but with no increase in gene copy number remains at 68%. For patients with an EGFR mutation-negative tumor, the response rate was low, irrespective of whether the EGFR gene copy number was high or normal. Lastly, in IPASS, the outcome with EGFR TKIs was better for patients with NSCLC who were selected on the basis of the presence of an EGFR mutation than it was for patients who were selected on the basis of the EGFR copy number. Therefore EGFR copy number should not be used to select patients for EGFR TKI treatment. It is not well established whether EGFR gene copy number has a predictive value for response to EGFR TKIs in selected patients with EGFR wild-type tumors. FISH-determined EGFR is currently being evaluated as a predictive biomarker for EGFR TKI therapy (i.e., cetuximab, necitumumab) in prospective studies.
EGFR protein expression by IHC has been assessed to predict response and outcome to EGFR TKIs. Total EGFR expression has not been shown to be associated with a better outcome to EGFR TKIs or with EGFR mutation. In a retrospective analysis, the use of a specific antibody targeting the intracellular domain of EGFR has been shown to improve the prediction of response to EGFR TKIs compared with antibodies targeting the external domain. Still, this method needs to be validated and does not appear to provide a better prediction than EGFR mutations can provide. Therefore total EGFR expression cannot currently be recommended to select patients for EGFR TKI therapy. However, high EGFR protein expression has been shown to predict response to anti-EGFR monoclonal antibody therapy (cetuximab). The American Society of Clinical Oncology guidelines include cetuximab plus chemotherapy as an option for first-line treatment of patients with tumors positive for EGFR expression by IHC based on the results of the First-Line ErbituX in lung cancer (FLEX) study. In the FLEX study update, there was a survival benefit for patients who had tumors with higher EGFR expression, when the score used considered the percentage of cells stained and the intensity of the staining (H score). Further evidence and validation in independent clinical trials are warranted to recommend the use of EGFR expression in select patients for therapy with an anti-EGFR monoclonal antibody.
Other Biomarkers of Resistance to EGFR TKIs
In addition to EGFR mutations, other biomarkers have been indicated as potentially associated with primary resistance to EGFR TKIs. KRAS mutations with constitutive activation of the downstream pathways are seen in 30% of people with lung adenocarcinomas and have been associated with poor prognosis. Based on retrospective analyses in trials of patients receiving EGFR TKIs as second- and third-line treatment, the presence of KRAS mutation is associated with lower response rates in patients taking EGFR TKIs (0% to 3%); however, there is no substantial effect on outcome. Because KRAS and EGFR mutations are considered mutually exclusive, KRAS testing is sometimes used as a screening assay and only KRAS wild-type tumors are tested for the EGFR mutation. This approach has not been validated and implies that enough material is available for successive testing of KRAS and EGFR mutations and would not delay the results. Therefore more data are required and KRAS mutation testing cannot be recommended to exclude patients for EGFR TKI therapy. Furthermore, although subtyping of KRAS mutations has been shown to have clinical relevance in colorectal cancer, no studies in lung cancer have established a clinically relevant difference between subtypes of KRAS mutations.
Mesenchymal–epithelial transition is another potential mechanism for resistance to EGFR TKIs. MET gene amplification has been associated with 10% to 20% of acquired resistance to EGFR TKIs. More recently, HER2 has been associated with acquired resistance to EGFR TKIs. High expression of insulin-like growth factor receptor 1 (IGF1R) has also been shown to be associated with resistance to EGFR TKIs. Recently, BCL2 interacting protein ( BIM ) polymorphism has been shown to potentially induce EGFR TKI resistance in patients with NSCLC harboring EGFR mutations. Currently, none of these biomarkers (MET, HER2, or IGF1R amplification) can be used for negative selection of patients for EGFR TKI treatment.
Patients Who Should Have Testing
Based on currently available published data, the EGFR sensitizing mutation is the only biomarker recommended as the predictive biomarker for testing of patients to receive EGFR TKI therapy. Although EGFR mutations are more frequent in tumors in patients who are Asian, female, or never-smokers, they also occur in other patients. Therefore clinical characteristics are not recommended for selecting or excluding patients for EGFR mutation testing.
EGFR mutations are most frequently found in people with adenocarcinomas and are very uncommon in people with pure lung squamous cell carcinomas and in pure small cell carcinomas (such as SCLC). However, mutations have been found in patients with other mixed carcinomas with an adenocarcinoma component, such as adenosquamous carcinomas or combined small cell carcinoma with an adenocarcinoma component. Consequently, in the absence of IHC evidence for the presence of an adenocarcinoma component, EGFR mutation testing is not recommended for patients with squamous cell carcinomas and SCLC carcinomas. In patients with marginally sufficient lung cancer specimens, including samples from biopsy, fine-needle aspiration, and cytologic samples from pleural effusions, the diagnosis of adenosquamous or poorly differentiated adenocarcinoma may be challenging, and an adenocarcinoma component should not be entirely excluded. This exclusion may explain why, in a few studies involving small specimens for EGFR mutation testing, EGFR mutations have been reported in rare cases of squamous cell carcinomas. In addition, in order to alleviate misclassification of squamous cell carcinoma versus adenocarcinoma of the lung on small specimens, the optimal IHC diagnostic algorithm should be used. The suitability of thoracic cytology for EGFR molecular testing has been confirmed, but IHC was used for histologic subtyping of the patients. Thus in cases of limited lung cancer specimens that may not exclude definitively an adenocarcinoma component, EGFR mutation testing should be performed in all patients, including those with squamous cell and small cell carcinomas. However, EGFR mutations have been identified in patients with proven squamous cell carcinoma of the lung. Therefore as recommended in the European Society for Medical Oncology guidelines and the CAP/IASLC/AMP guidelines, molecular testing could be performed in patients with squamous cell carcinoma of the lung who are never-smokers and former light-smokers (fewer than 15 pack-years). EGFR mutation testing should also be performed for patients with tumors that are classified as NSCLC not otherwise specified (NOS), if possible.
When Testing Should Be Done
Evidence for the use of EGFR mutation testing in selecting patients for EGFR TKI therapy is available only for those with advanced NSCLC. By contrast, evidence for its use to select patients for adjuvant TKI therapy in early-stage and surgically treated disease is currently not available. The test is not useful for many of these patients who will have surgery with or without adjuvant chemotherapy. However, for 50% of patients who are expected to have relapse, no initial testing on the resected specimen may mean that the test must be done at the time of disease progression, with delayed availability of the result, lack of a readily available sample, and even the necessity of repeating biopsy. Thus clinicians must balance the cost of performing unnecessary tests in cured patients with delaying therapy in others because results are not readily available. At the time of relapse, it is also relevant to consider the value of EGFR mutation on the diagnostic sample and the possibility of resistant mutations present in the metastatic relapse sites but not in the primary tumor.
Tumor Site to Be Used for Testing
The type of samples used for EGFR testing is largely determined by the convenience of sample availability. Currently, testing of the primary tumor or metastatic lesions is equally acceptable before initial EGFR TKI treatment. However, there is some debate with regard to sample choice for testing because of tumor heterogeneity. In some studies, EGFR mutation testing has been shown to be very consistent between the primary lung tumor and metastatic lesions. However, other investigators have reported heterogeneity of the EGFR mutation status between the primary lung tumor and the metastasis. Overall, the quality of the tissue should be the primary factor for choosing the sample for a patient with metastatic lung cancer. Nevertheless, as previously discussed, metastatic bone lesions might not be optimal for testing if the biopsy specimen has been processed in acidic decalcifying solutions. For patients with multiple primary sites, it seems rational to test each tumor separately, as the detection of different mutations in different primary tumors has been reported.
Clinical Recommendations for EGFR Testing
The guideline by CAP/IASLC/AMP recommends that, at diagnosis, any patient with advanced NSCLC with an adenocarcinoma, a large cell carcinoma, or a carcinoma with an adenocarcinoma component should be tested for the EGFR mutation, using the most accessible tissue (primary tumor or metastasis). If the specimen is not large enough to exclude an adenocarcinoma component, other histologies, such as squamous cell carcinomas and small cell carcinoma, should be considered for EGFR mutation testing as well. For patients with early-stage NSCLC, EGFR mutation testing at the time of diagnosis is debatable, but seems reasonable and should be done if possible.
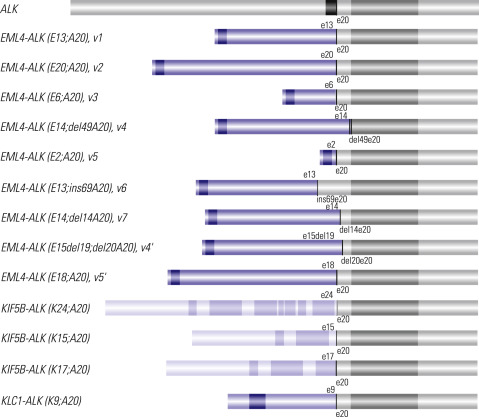
ALK Rearrangement: A Predictor of Response to ALK Inhibitors
The other approved targetable biomarker used in the treatment of patients with advanced lung cancer is ALK gene rearrangement. The discovery in 2007 of the ALK gene rearrangement in lung cancer has been quickly translated into a therapeutic target. The most frequent ALK rearrangement is an inversion on the short arm of chromosome 2 resulting in a fusion gene, echinoderm microtubule-associated protein like 4 (EML4)–ALK , of which the fusion protein product demonstrates a constitutive tyrosine kinase activity. In addition, other fusion partners of ALK have been reported in lung cancer, including kinesin family member 5B (KIF5B)–ALK and TRK-fused gene (TFG)–ALK, which are rare fusions involving a translocation with a chromosome segment other than 2p ( Fig. 18.3 ). The prevalence of ALK fusion ranges from 2% to 7% of patients with NSCLC ( Table 18.5 ). ALK rearrangements appear more frequently in never-smokers and, potentially, in younger people, and occur more often in adenocarcinomas than in other NSCLC histologic types. However, ALK rearrangements seem not to be associated with gender or ethnicity, in contrast to EGFR mutations.