Molecular Prognostic Markers of Gastric Cancer
Mahmoud Goodarzi
Dongfeng Tan
INTRODUCTION
According to a recent global estimate of cancer incidence, stomach cancer is the second most frequent cancer-related cause of death after lung cancer. Although the worldwide incidence of gastric cancer has been declining in recent years, its mortality rate in China is the highest among all tumors and represents 25% of gastric cancer mortalities worldwide.1 Though advances in diagnosis and treatment have provided significantly improved long-term overall survival (OS) rates for early gastric cancer, the prognosis for advanced gastric cancer still remains poor.
Many factors are associated with gastric cancer prognosis, including the location of the tumor in the stomach, histological grade, and lymphovascular tumor invasion. For instance, Asian race, female sex, and younger age are predictive of a better outcome, while signet ring cell carcinoma of the stomach is reported as a major and independent predictor of poor prognosis due to specific characteristics such as more infiltrating tumors showing affinity for lymphatic tissue accompanied by a higher rate of peritoneal carcinomatosis.2
Nevertheless, among all factors, regional lymphatic spread and disease stage are probably the most powerful prognostic factors determining the survival, management, and prognosis of patients with stomach cancer. Currently, surgical resection remains the most reasonable treatment option for stomach cancer; tumor node metastasis (TNM) stage (by AJCC Cancer Staging Manual, see Chapter 18), which is assessed after tumor resection, is used as the main prognostic factor,3 with 5-year survival rates of 58% to 78% and 34% for stage I and II disease, respectively1 However, it is not rare that stomach cancer patients with the same TNM stage, receiving the same integrated treatments, have different clinical outcomes. Therefore, new prognostic determinants capable of selecting patients at high risk of treatment resistance in conjunction with the TNMstaging information are required.
Extensive research in past 2 decades has increased our understanding of the biological mechanisms of tumor invasion and metastasis, which are critical determinants of clinical behavior of cancer. Tumor invasion and metastasis is a very complicated process, involving a multistep progression through a series of sequential selective events.3 It has been documented that metastatic process involves many related steps, including tumor cell detachment, local invasion, motility, vascular invasion and neoangiogenesis, access to and survival in circulation, adhesion to endothelial cells, extravasation, settlement and regrowth in different organs.3 At the molecular level, multiple genes regulating these events contribute to the metastatic process, including cell cycle regulators, cell adhesion molecules, protein catabolic enzymes, and various angiogenic and growth factors, which are often used as prognostic factors.1,3 Better knowledge of the molecular basis for tumor metastasis will lead to new paradigms and possible improvements in
diagnostic and therapeutic approaches. This chapter describes changes in genes and molecules that can be used as prognostic markers of gastric cancer and their potential application in the clinical setting.
diagnostic and therapeutic approaches. This chapter describes changes in genes and molecules that can be used as prognostic markers of gastric cancer and their potential application in the clinical setting.
MOLECULAR PROGNOSTIC MARKERS OF GASTRIC CANCER
Tumor-infiltrating Lymphocytes in Gastric Cancer
Cell-mediated adaptive immunity is thought to play a major role in antitumor immunity. In mouse models using transgenic or knockout mice or applying monoclonal antibodies specific for distinct immunologic components, adaptive immunity has been demonstrated to protect murine hosts against the development of both chemically induced and spontaneous tumors.4 Several types of tumor-infiltrating lymphocytes (TILs) have been shown to be associated with better outcomes for different human cancers, such as melanoma and colorectal cancer.4 This study demonstrated that high numbers of CD3+, CD8+, or CD45RO+ T cells in tumor tissue are significantly correlated with lower frequencies of lymph node metastasis or disease recurrence and improved patient survival. Lee et al.4 have shown that the type and density of TILs correlate with clinical outcome after gastrectomy in stomach cancer patients. A high density of CD3+, CD8+, or CD45RO+ tumor-infiltrating total T lymphocytes (cytotoxic T cells and memory T cells) was found to be an independent predictor of lymph node metastasis and an independent prognostic factor for patients’ OS by multivariate analyses, suggesting that adaptive immunity does act to prevent tumor progression and helps predict clinical outcome.4
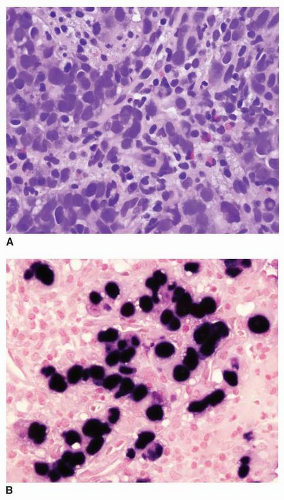
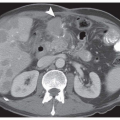
EBV (Epstein-Barr virus)-associated gastric cancer (EBVAGA, see Chapter 4) is frequently seen in medullary type or lymphoepithelial type of gastric cancer, with dense lymphocyte infiltration.5 EBVAGA is also seen in other types of gastric cancer, including intestinal-type gastric adenocarcinoma. Nevertheless, one of the main clinical features is that EBVAGA is associated with a favorable clinical outcome. Though it is still not quite clear the underlying mechanism(s), infiltrating lymphocytes, particularly T lymphocytes, seem to play a role in limiting the spread of cancer cells.6 An intestinal-type EBVAGA with intratumoral T lymphocytes is illustrated in Figure 17-1.
Cell Cycle Regulators
Gastric carcinoma is characterized by numerous genetic and epigenetic alterations that may influence cell cycle progression and apoptosis.7 Abnormal expression of cell cycle regulatory proteins that control the G1-S phase transition, a critical rate-limiting step in cell cycle progression, is frequently observed in tumorigenesis.8 These proteins include three D-type cyclins (D1, D2, and D3) that bind to one of two cyclin-dependent protein kinase (CDK) subunits, CDK4 and CDK6, as well as the E-type cyclins, which control the activity of CDK2.8 It has been shown that the cyclin E gene is amplified in 15% to 20% of gastric cancers and overexpression of cyclin E correlates with the aggressiveness of the tumor.3
The P21 and P27 tumor-suppressor genes produce proteins that are activated by P53 and induce cell cycle arrest by inhibiting the kinase activity of the cyclin/CDK complexes regulating cell cycle progression.9 A reduction in the expression of P27Kip1 is frequently associated with advanced gastric cancers and is also significantly correlated with deep tumor invasion and lymph node metastasis.3 Furthermore, it has been demonstrated that P21 expression, alone or in combination with P27, is associated with a favorable prognosis and is an independent predictor of patient survival in gastric cancer.7 Moreover, the tumor suppressor gene P53 encodes a nuclear protein that plays a key role in tumor progression by regulating DNA repair, cell division, and apoptosis. P53 induces the expression of P21, which promotes cell cycle arrest by inhibiting the phosphorylation of the CDK complexes and blocking cell cycle progression to the S-phase.10
The prognostic usefulness of P53 in gastric cancer has been controversial; most studies show an association of P53 with patient survival, while other investigations do not support these
findings.7 These conflicting reports may be explained in part by the investigators’ use of different techniques and antibodies in the studies.9 Fondevila et al.11 found that P53 expression is an independent prognostic factor for both disease-free survival and OS in patients with curatively resected gastric cancer, and when tumor cells express P53, the therapeutic efficacy of adjuvant chemotherapy is lost.
findings.7 These conflicting reports may be explained in part by the investigators’ use of different techniques and antibodies in the studies.9 Fondevila et al.11 found that P53 expression is an independent prognostic factor for both disease-free survival and OS in patients with curatively resected gastric cancer, and when tumor cells express P53, the therapeutic efficacy of adjuvant chemotherapy is lost.
Despite the controversy regarding the usefulness of P53 as a prognostic marker for gastric cancer, the combined P53/P21 status may be a useful prognostic marker for survival in patients with surgically resected gastric cancer.10 In fact, expression of P21 in combination with a lack of P53 expression was significantly associated with longer survival.7,10 In addition, Liu et al.12 suggested that the combined examination of P27, P21, and P53 expression allows the precise prediction of prognosis in patients with gastric cancer. Therefore, the simultaneous evaluation of the expression of P53, P21, and P27 may be a useful diagnostic tool for predicting clinical prognosis in patients with stomach cancer.7,10
The Rb Pathway
The retinoblastoma tumor suppressor (Rb-1), P16 (CDKN2/MTS1), and cyclin D1 genes are components of the Rb pathway, which controls the G1/S checkpoint of the cell cycle.13 The active hypophosphorylated form of the Rb protein binds to and blocks the action of the transcription factor E2F, inhibiting the progression of the cell cycle from the G1 phase to the S phase. Cyclins D1 and E, in association with their cyclin-dependent kinase partners, are responsible for the phosphorylation of Rb.3,13 P16 binds to CDK4 and CDK6, blocking their association with D-type cyclins. Thus, inactivating mutations or deletion of Rb or P16 with loss of protein expression or overexpression of cyclin D1 or cyclin E can activate E2F and promote cellular proliferation.14 Survival studies suggest that most gastric adenocarcinomas exhibit abnormal expression of at least one of the pRb, P16, or cyclin D1 genes, raising the possibility that most gastric cancers harbor Rb pathway abnormalities.13 Univariate and multivariate survival analyses have revealed that reduced expression of Rb is associated with worse OS.13,14 Furthermore, it has been shown that Rb expression is lower in lymph node metastases than in the corresponding primary tumors. However, P16 and cyclin D1 overexpression had no prognostic value for gastric cancers in those studies.13
Cell Adhesion Molecules
E-cadherin, which is encoded by the CDH1 gene, is a 120-kDa transmembrane glycoprotein that is responsible for calcium-dependent homotypic intercellular adhesion,15




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree