Fig. 21.1
MMS technique (1). Debulking. The visible tumour is debulked with scalpel (2). Excision of 1–2 mm of healthy tissue surrounding the wound resulting from debulking (3). The specimen is sectioned and marked with ink on its cut edges. Each section is individually marked in its entire perimeter (4). Mapping. The segments are numbered and the tissue is oriented according to a map or diagram representing the anatomical area from where the tissue is excised (5). The tissue sample is flattened and sliced in the microtome. Proper flattening makes it easier to obtain sections that enable the epidermis to the deep dermis in a single specimen (6). Histopathological interpretation (7). Mapping of residual tumour. The location of the residual tumour revealed under the microscope should be drawn on the Mohs map (8). Subsequent Mohs stages. A next layer should be taken from where the residual tumour resides (9). Sectioning and inking as in number 3. Repeat steps 4, 5 and 6, until tumour-free margins are histologically confirmed
Frozen-section study is both more immediate and more interactive than microscopic readings of paraffin-embedded tissue. It also allows for immediate reconstruction, if required, in full confidence that there is no residual tumour. Horizontal sectioning compared to vertical sectioning saves time and also minimises the possibility of false negatives, as it requires fewer specimens for a comprehensive view of tumour margins from the epidermis to the deep dermis [9].
When doubts arise over fresh-tissue readings, MMS is performed in paraffin-embedded sections. This approach, called slow Mohs, requires more time; however, although it loses in interactivity, it gains in terms of enhanced margin control [10]. Some tumours require special immunohistochemical staining to identify tumour cells in Mohs surgery margins. Different immunostains have proved useful in detecting tumour cells in a number of malignancies, including melanoma. Furthermore, it is now possible to use rapid stains for certain melanoma antigens in frozen sections with the same reliability as permanent paraffin-embedded sections [11]. Below we review each of the MMS stages in more detail.
First MMS Stage
Harvesting
MMS commences when the Mohs surgeon removes tumour tissue. It is important to first delimit the area well, however, bearing in mind the subsequent steps until a histologic section is obtained. Tissue removed en bloc with a bevel angle of 45 % will be easier to process later. It is also important that the histotechnician is present when the tissue is excised. Many surgeons debulk the tumour before commencing MMS, as debulking enables more accurate definition of the clinical margins, eliminates the possibility of false positives and facilitates the teasing down of the peripheral margins so that complete sections can be obtained without loss of epidermis [4, 12]. The first Mohs stage involves excision of 1–2 mm of healthy tissue surrounding the clinically visible tumour or the wound resulting from debulking. On excision, the surgeon makes a notch as a reference point for the tissue, typically at 12 o’clock. For specimens measuring more than 2 cm, another reference notch is made at 3 o’clock or coinciding with a specific anatomical fold or crease.
Processing
The tissue is oriented according to a map or diagram representing the anatomical area from where the tissue is excised. The surgeon places the tissue on the map and makes a drawing that matches the resected specimen in size and location. A photograph can also be used for this purpose. Working with the histotechnician, the surgeon cuts a minimum number of segments that are small enough to fit on the slides and be cut by the cryostat in a single section. The segments are numbered and the borders of each section are inked. Different colours are used to represent the epidermis to the deep dermis, with the result that each section is individually marked in its entire perimeter. The colours used should correspond to those used in the diagram.
The tissue is next prepared for sectioning in the cryostat. Different methods have been described to obtain histologic sections that can be observed across the entire margin of specimen previously in contact with the patient. The best-known approaches include the heat extractor technique with an OCT embedding medium, microscopic glass slides and mechanical flattening techniques with liquid nitrogen [13, 14]. This sectioning step is crucial, and irrespective of the system used, success depends greatly on the experience of the histotechnician. Proper flattening makes it easier to obtain sections that enable the epidermis to the deep dermis of a single specimen to be viewed.
The obtained sections are then stained, most commonly with H&E; toluidine blue is sometimes preferred for basal cell carcinoma (BCC), as it highlights the mucopolysaccharide stroma in metachromatic pink [15].
Histopathologic Interpretation
Even if an experienced pathologist makes the histologic readings, the Mohs surgeon needs to view each and every slide. The Mohs surgeon first scans the slides to make sure that the sections are fully represented and to check that inking is complete, the epidermis is completely visible, the notches correspond to the map, the colour of the stain is appropriate and that there is no loss of tissue that could result in false negatives [16]. Magnification with a ×2 objective lens is usually sufficient for this purpose. If any of the sections are observed to be defective, recuts should be made to obtain a suitable specimen. The Mohs surgeon next looks for evidence of residual tumour in the margins. Failure to find cancer cells means that MMS is complete. If tumour cells are identified, they are carefully drawn on the diagram, which is used to guide the next MMS stage.
Subsequent MMS Stages
As in the first stage, the Mohs surgeon proceeds to remove 1–2 mm of margin coinciding with the map drawing. Cancer foci located in the epidermis or dermis require the defect to be extended laterally, whereas persistent neoplasms in subcutaneous fat or underlying structures may only require extending the excision in a deeper plane [4]. It is therefore important for the Mohs surgeon to view the histologic sections under the microscope to know the exact distribution and location of residual tumour involvement. Once histology readings confirm negativity, the type of reconstruction can be decided and MMS is complete.
Practical Considerations and Applications
Although MMS in some cases is performed under general anaesthesia in hospital, the vast majority of surgeries are performed in outpatient settings under local or locoregional anaesthesia with sedation. The dermatological oncological surgeon specially trained in the Mohs discipline usually has a team of assistants (trained nurses, histotechnicians, etc.) to hand, including, as necessary, specialists such as pathologists, plastic surgeons, otolaryngologists and ophthalmologists. The success or failure of MMS depends on effective coordination of each step in the procedure. Some practical considerations based on our own experience are described below, all aimed at maintaining cure rates while saving time and as much healthy tissue as possible.
Preoperative Evaluation
The patient should be an appropriate candidate for MMS. In the initial screening visit, it is advisable to ensure that the patient understands MMS and is aware of the medical, functional and aesthetic outcomes. It is also desirable, depending on the location and extent of the tumour, to perform imaging and other studies and to consult with other specialists if they are to be called on during the procedure.
First MMS Stage
The ideal MMS is performed in a single stage, as maximum efficiency is achieved when the first stage is the last and the tumour is accurately delimited so that no more tissue than necessary is removed. As mentioned above, it is important to debulk the tumour prior to implementing the first stage, as this minimises the number of stages, reduces false positives and helps flatten the specimen [13]. Mohs surgeons typically use curettage for tumour debulking; in our department, however, we have a preference for using a scalpel for several reasons. The scalpel is cleaner than the curette as it leaves no impurities that can sometimes cause floaters [17]. It can also be used in locations where curettage is difficult, for example, the free edge of the eyelids and the mucosa of the lips. However, the most important reason is that the scalpel, unlike the curette, preserves the debulked tissue for histologic processing together with first-stage tissue. In our laboratory, vertical cuts of debulked tissue are processed together with sections obtained in first-stage Mohs, resulting in biopsies of tumour tissue with the same stain as the Mohs specimen. Thus, if we have any doubt regarding the existence of residual tumour in a Mohs specimen, we can check whether the suspect cells have the same configuration, layout, colour and cellularity as those in the biopsied debulked tissue. This comparison is more reliable than comparison with a previous paraffin-embedded biopsy that uses a different stain. It also means that we can send tumour tissue for paraffin-embedded study if in doubt or if a previous biopsy is unavailable (Figs. 21.2, 21.3, 21.4, 21.5 and 21.6).
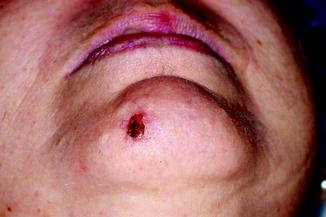
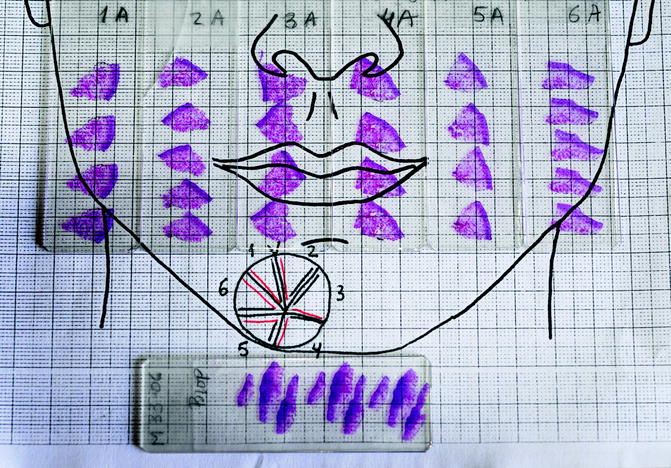
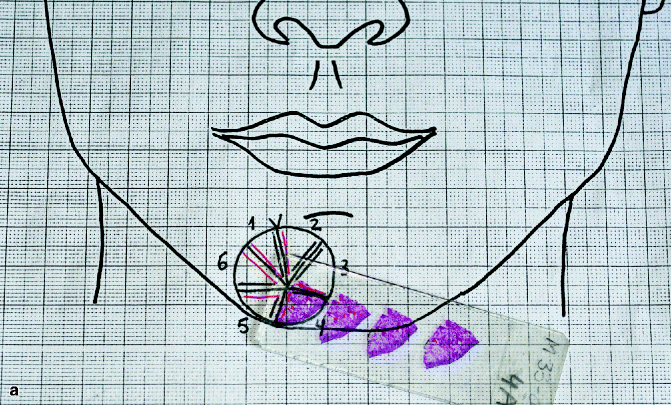
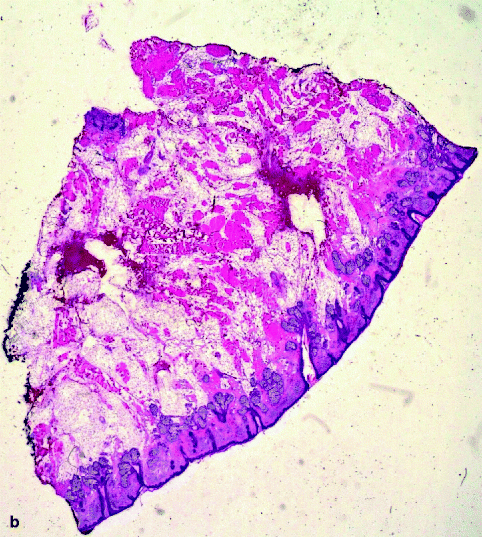
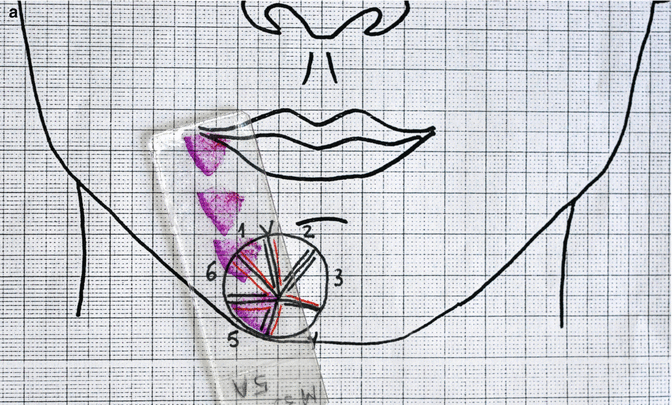
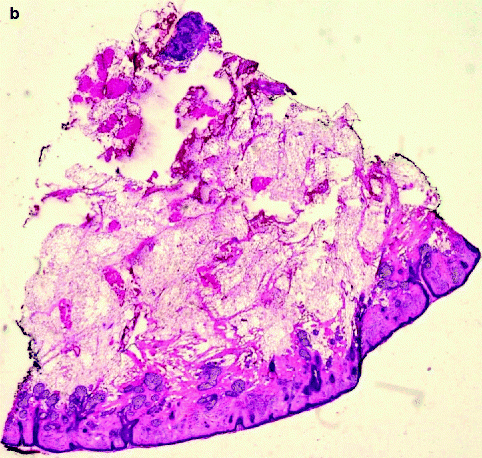

Fig. 21.2
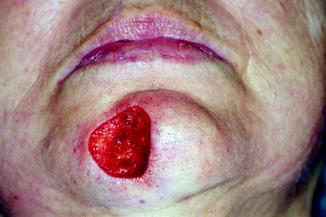
Morpheaform basal cell carcinoma on the chin. The lesion is very ill defined

Fig. 21.3
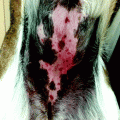
First stage of Mohs surgery for lesion in Fig. 21.2. Note the biopsies (vertical cuts) of the debulked tumour together with all sections obtained in first Mohs stage


Fig. 21.4
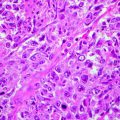
(a) Slide corresponding to number 4. The slide is on the map. The sections match in size and location. (b) Residual basal cell carcinoma persists at the deeper margin. Section of Fig. 21.4a (haematoxylin and eosin, original magnification ×2)
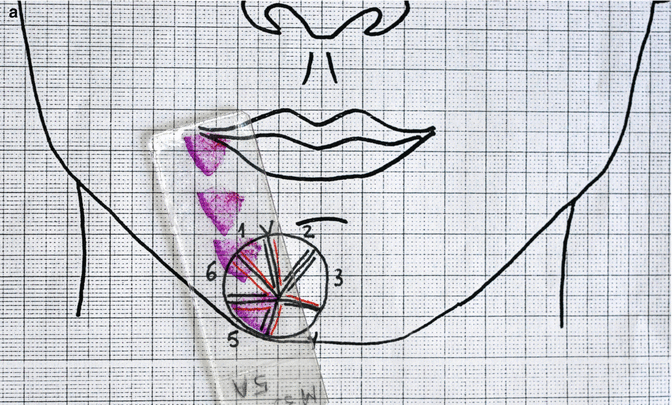

Fig. 21.5
(a) Slide corresponding to number 5. (b) Remaining basal cell carcinoma is observed on examination of the histopathology deep in the corner close to the previous section (haematoxylin and eosin, original magnification ×2)
Although debulking is aimed at removal, delineating the actual tumour is clinically difficult. A number of studies have described different approaches to delineating tumour boundaries prior to MMS, including Doppler ultrasound, confocal microscopy and, more recently, aminolevulinic acid-induced fluorescence in photodynamic diagnosis; [18–20] these approaches, however, do not represent any advance over clinical judgement.
Delimiting the tumour may be problematic yet it is clinically the most important step in terms of saving time during MMS. The tumour to be removed often persists after previous treatments failed to eradicate the tumour in its entirety. In such cases, as well as excising the tumour, the scar must also be removed, as it may contain contiguous tumour remains. Scars resulting from previous treatments (surgery, radiotherapy, curettage, cryotherapy, imiquimod, etc.) should also be removed as they may conceal residual tumour beyond the unaffected tumour margins. In the interest of ensuring a cure, we cannot run the risk of tumour recurrence due to remains excluded by MMS.
In the remaining histologic sectioning steps in our department, the Mohs surgeon and histotechnician work together, with the surgeon responsible for preparing, grossing and inking the tissue, map drawing and specimen orientation, and the histotechnician responsible for sectioning, embedding, cutting and staining. This close collaboration enhances efficiency and minimises the possibility of the most common errors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree