The genesis of overt metastases in breast cancer is based on the idea that tumor cells that dissociate from the primary cancer get access to circulation either directly into blood vessels or after transit in lymphatic channels. Thus detection of such cells in patients with newly diagnosed solid tumors has been an appealing strategy to provide evidence for future metastases.
In the past, several models have been constructed to explain the presence of individual tumor cells in secondary organs and their influence on the subsequent course of the disease. Currently, according to most recent transcriptome and genome analyses, tumor cells circulating in the bloodstream (CTCs) and those already disseminated to secondary organs (DTCs) are viewed as rare and much earlier indicators of tumor cell spread than generally assumed from the typical year-long course of cancerous diseases, such as breast cancer. Despite the observation that the numerous genetic alterations found so far in such cells are rarely identical or even similar, the idea that some of these cells might be progenitor cells with self-renewing properties that give rise to most of the tumor mass that is dealt with clinically is supported by the following: (1) the long interval between dissemination and clinical manifestation of metastases, (2) the frequently observed relative resistance of some cells to chemotherapy, and (3) their significant effect on disease progression, despite their low abundance in secondary organs.
Beyond the discussion of such models and opinions, the actual presence of tumor cells outside the primary tumor and in organs relevant for subsequent metastasis formation, such as bone and bone marrow, would serve 3 purposes that could be clinically useful:
As unambiguous evidence for an early occult spread of tumor cells
As a relevant risk factor for subsequent metastasis and thus a poor prognosis
As a marker for monitoring treatment susceptibility
The 2 main approaches for the assessment of minimal residual cancer—both for DTCs and CTCs—are immunologic assays using monoclonal antibodies directed against histogenetic proteins and polymerase chain reaction (PCR)–based molecular assays exploiting tissue-specific transcripts. In review of the last decades, the immunologic approach emerged in the first place due to the technological availability, whereas molecular techniques seem to have taken over in the last few years.
Tumor cells evaded or shed to the blood circulation may be detectable in peripheral venous blood and, in principle, all body organs, as shown in a few elegant experiments during the 1960s and 1970s.1,2 When specific monoclonal antibodies became broadly available in the 1980s, the interest in the identification of spread tumor cells was renewed. The first study groups,3-8 however, did not investigate peripheral blood, presumably due to pathophysiologic considerations, the presence of tumor cells in circulation was thought to be merely temporary. For similar theoretical reasons, the initial studies did not investigate epithelial parenchymous organs, such as lung, liver, or brain, known to be frequently colonized by epithelial cancer cells, because specific antibodies available at that time largely only discriminated between the histogenetic origin of cells, although they were raised against tumor-associated antigens. The ability to detect nonautochthonous epithelial breast cancer cells in a mesenchymal organ environment, characterized by a physiologic absence of epithelial cells, its relevance for breast cancer metastases, and the clinical convenience to explore (ie, diagnose) the organ site of interest led to numerous studies investigating the presence and significance of hematogenously disseminated tumor cells in bone marrow samples.9-18
Protocols for the direct analysis of unprocessed samples exist, but most approaches require an enrichment of DTCs and/or CTCs before application of the detection technology. Enrichment is usually based on either density-gradient centrifugation or immunomagnetic procedures19,30 since its introduction in the early 1980s20 and validation thereafter.21-23
Of particular note, we have to appreciate the varying quality of immunologic stainings, as we learned our lessons from the HER-2 and the hormone receptor stories during the past decade. Nevertheless, the first question that arises is that of the putative origin of the detected cells, as “positive” staining may have multiplex reasons. The strongest and most likely unequivocal reason to accept that DTCs are of epithelial origin stems from analysis of control samples. Among various series of either healthy candidates or patients with benign disease conditions, reports of positively stained cells usually aroused skepticism on the validity of the approach. Sorting out unspecific staining by both adherence to morphologic criteria and exclusion of immunologic artifacts (both factors we address in details below), the true false-positive rate ranges between 1% and 5% in the hands of experienced and devoted laboratory experts.14,24-26 The currently accepted benchmark technology for the detection of DTCs and CTCs implies Ficoll-Hypaque density centrifugation, collection of mononucleated cells from the interphase layer, and subsequent preparation of cytospins by using a cytocentrifuge, most often applying 5 to 10 × 105 mononucleated cells per slide, followed by immunostaining for epithelial cells on cytospins. Crucial steps in this process are sampling techniques and the methods used for immunostaining, including the choice of antibodies.27,28 The proof of concept and the demonstration of the clinical utility of this approach has been shown in a recent pooled analysis.29 The most widely used monoclonal antibodies are A45-B/B331 and AE1/AE3,32 for which clinical significance is well documented in numerous clinical trials.17,29,33-36
The alkaline phosphatase anti-alkaline phosphatase (APAAP) visualization system,37 in combination with the red chromogen New Fuchsin, represents the immunostaining method most widely used. It is known that false-positive staining of hematopoietic cells (HCs) does occur.24 Mechanisms for such unintended reactions include direct binding of alkaline phosphatase (as part of the APAAP detection system) to plasma/preplasma cells,26 cross-reactivity of the primary anti-epithelial antibodies with HC epitopes, and interactions with Fc receptors present on leukocytes. Morphologic evaluation of immunocytochemistry-visualized cells is feasible25,38; however, it is noteworthy that morphologic overlap between tumor cells and false-positive HCs exists.24-26,39 To exclude such unspecific interference, high-quality approaches usually incorporate parallel immunostaining in which, for control purposes, additional sample slides are incubated with immunoglobulin isotype-identical antibodies directed against irrelevant antigens. Figure 27-1 exemplifies the analytical algorithm according to guidelines implemented by the European International Society for Hemotherapy and Graft Engineering (ISHAGE) Working Group for Standardization of Tumor Cell Detection.25 The clinical validation on 817 patients with breast cancer33 confirmed that only cells classified as “tumor cell” or “probable tumor cell” (Fig. 27-1A through 27-1D), and tumor cells despite signs of degeneration (Fig. 27-1I and 27-1J) signified poor prognosis, as compared with presence of cells classified as HC (Fig. 27-1E through 27-1H).
Figure 27-1
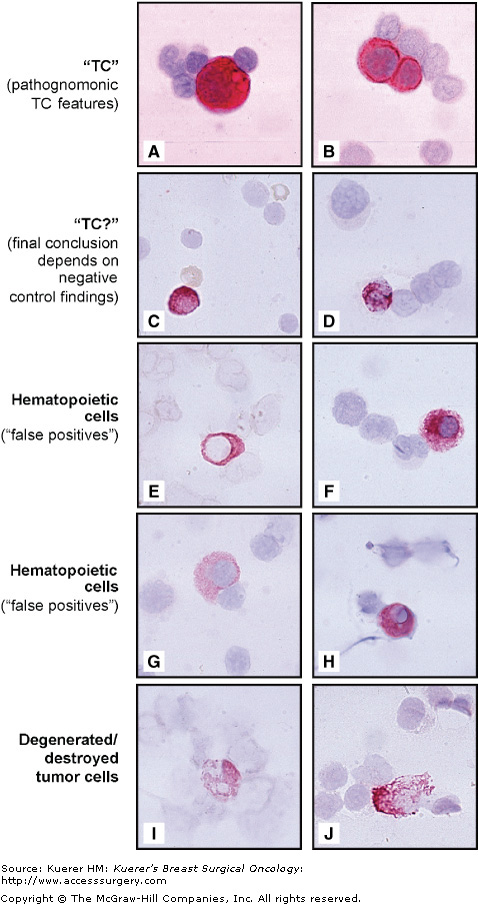
Categorization of immunostained cells for the detection of breast carcinoma cells in bone marrow and peripheral blood. “Tumor cell,” pathognomonic features of epithelial tumor cell nature, with a clearly enlarged nucleus compared with the size of neighboring hematopoietic cells (A) and/or the formation of clearly immunostained tumor cell doublets/clusters (B), a morphology never observed in false-positive hematopoietic cells or in negative control specimens. “Probable tumor cells” represent a morphologic overlap between tumor cell and hematopoietic cell, lacking pathognomonic features of tumor cells, but morphologic signs of hematopoietic cell are also absent; typically cytoplasmic staining is strong (C,D), often irregularly distributed (D), and clearly overlaying the nucleus (C,D). “Hematopoietic cells” (so-called false positives): typical hematopoietic cell features include a small, hematopoietic cell-sized nucleus (E–H) with an even or regularly distributed cytoplasmic staining (G,H), which often is microvacuolar (G) and not overlying the nucleus (E,G,H); a small, pin-point vacuole is typically seen in the hematopoietic cell cytoplasm (H), whereas larger vacuoles may be present in actual tumor cells (A). “Destroyed/degenerated tumor cells” are tumor cells that show morphologic signs of degeneration and or destruction (I,J). (Adapted and reproduced with permission from Borgen E, Naume B, Nesland JM, et al. Standardisation of the immunocytochemical detection of cancer cells in bone marrow and blood: I. Establishment of objective criteria for the evaluation of immunostained cells. Cytotherapy. 1999;1:377-388. and Naume B, Wiedswang G, Borgen E, et al. The prognostic value of isolated tumor cells in bone marrow in breast cancer patients: evaluation of morphological categories and the number of clinically significant cells. Clin Cancer Res. 2004;10:3091-3097.)
PCR methods targeting tissue-specific gene expression initiated a competitive race against immunocytochemistry for the detection of DTCs and CTCs in the beginning of the 1990s.40-42 PCR-based assays are extremely sensitive and are able to detect a single cell in a sample of 2 × 107 or more white blood cells.43 However, a few transcripts—for example, by either illegitimate low-level expression of the targeted mRNA in hematopoietic cells44 or amplification of DNA-reassembling sequences45 (“pseudogenes”), down-regulation of the actual gene in DTC/CTC, and possibly the presence of rare normal epithelial cells46,47—cause false-positive signals in noncancer controls, and only since the introduction of the quantitative real-time PCR (qPCR) methods has this problem been addressed.48 In view of the lack of true cancer-specific molecular targets, qPCR became more or less the state-of-the-art quantitative method, allowing one to determine cut-off values of marker transcript numbers in samples of noncancer controls,49-51 above which transcripts can be considered as tumor cell–derived. Moreover, the expression level of all known marker genes varies between tumors from different patients and even among cells of the same tumor. This cancer heterogeneity points to the use of multiple marker mRNAs.57,58 Consequently, the discovery of sensitive mRNA markers has been approached using specific differential gene expression screening of primary tumors and normal tissue. The technical problems have been substantially reviewed and are beyond the scope of this section.19,48,52 Nevertheless, recent data suggest prognostic significance for molecular CTC detection in breast cancer patients in various stages,53-55 including patients with lymph node–negative disease.56
In order to investigate the underlying biology of DTCs and CTCs, antibodies against tumor-associated antigens—in parallel with the (usually anticytokeratin) detection antibody—are used to profile these cells, predominantly to, among various reasons, determine what percentage of these cells are actually of neoplastic origin. Using such immunocytochemical procedures, it has been possible to identify frequent expression of urokinase-type plasminogen activator receptor (uPAR) in gastric cancer59 and HER-2 (also known as ERBB2 or HER-2/neu) in breast cancer (Meng, 2004 #1582; Meng, 2004 #1584; Solomayer, 2006 #1627).60 In both cases, the (over) expression was associated with poor clinical outcome, and uPAR and HER-2, as a consequence, might be important for the survival and growth of DTCs.59,60
Detailed examination of the genome was made possible by recent technical developments, such as the combination of immunocytochemistry and fluorescence in situ hybridization, which led to the demonstration that the bone marrow contains disseminated epithelial cells of malignant origin.61 By developing a new procedure for whole-genome amplification and subsequent comparative genomic hybridization of single immunostained cells, Klein and coworkers demonstrated that cytokeratin-positive cells in the bone marrow of patients with epithelial breast cancer without clinical signs of overt metastases (stage M0) are genetically heterogeneous.62 This heterogeneity was strikingly reduced with the emergence of clinically evident metastasis (stage M1). Similar to these genomic analyses of DTCs, research groups showed the malignant nature and viability of CTCs from peripheral blood samples.38,63-67
The stage at which individual cells leave the primary tumor is unclear. In patients with early-stage invasive breast cancer, the cytokeratin-positive cells isolated from the bone marrow had few features in common with those found in their respective primary tumors.68 A provocative interpretation of this surprising finding is that the DTCs separated from their primary tumor at a very early stage. This hypothesis is also supported by the finding that only a few DTCs in these patients had TP53 mutations, which are associated with the later stages of tumorigenesis.62,69 DTCs might therefore evolve independently into overt metastases, driven by the specific selective pressures of the bone marrow environment.70
Genetic analyses that compared paired primary and metastatic breast tumor samples confirm the hypothesis that DTCs evolve independently from the primary tumor. The patterns of genetic alterations that are observed in overt metastases are often discordant with those of the primary tumor and differ almost completely in approximately one-third of the cases.71 During genetic progression of the primary breast tumor, cancer cells might disseminate continuously, acquiring additional genetic alterations after migration into secondary organs such as the bone marrow.
The advent of targeted therapy in oncologic treatment of patients with breast cancer was praised as the major momentum of the last decade. Surprisingly enough, review of the literature and both ongoing and completed clinical trials show the discrepancy between pretension and reality. The approaches rarely ever seek to demonstrate that the target is present on residual tumor—it is without saying that assessment of protein expression of primary tumor cells does not fulfill the criteria to call a patient “target-positive” because neither residual tumor cells may be present nor residual tumor cells may express the target. The data for the latter hypothesis stem from DTC and CTC characterizing studies. The most prevalent therapeutic targets—such as estrogen receptor and HER-2—are heterogeneously expressed in DTCs and/or CTCs, and extrapolation from the corresponding primary tumor to the spread tumor cell pool usually failed to confirm concordance. Fehm and colleagues72 showed that primary tumor cells and DTCs had a concordant estrogen receptor status in only 28% among 38 patients with DTCs in bone marrow and an estrogen receptor–positive primary tumor. Similarly, Schardt et al73 and Solomayer et al65 recently showed in their molecular and immunologic analyses, respectively, that a concordant HER-2 status may be observed in as few as 4% of the patients. In addition to these data, Balic et al74 analyzed similarities between the proposed stem cell phenotype and the phenotype of DTCs in breast cancer patients. The results indicated that almost all patients with DTCs have stem cell–like DTCs in the detectable pool of their DTCs and, moreover, that the majority of DTCs have a stem cell–like phenotype.74 These findings support the previous data on both estrogen receptor negativity and HER-2 heterogeneity, which are usually found in tumor cells with stem cell or progenitor cell characteristics.
Obviously, such data are strong arguments to explain failure of obviously effective treatment strategies for which efficacy has been demonstrated in clinical trials of metastatic breast cancer patients in which—at least sometimes—the biology of the metastatic lesion is assessed, but where the response is almost always monitored according to Response Evaluation Criteria in Solid Tumors (RECIST). This principle has been left out of adjuvant treatment approaches, most likely because of the lack of (or perhaps ignorance to) an appropriate surrogate marker.
Given the clinical evidence for the prognostic importance of viable tumor cell dissemination, the emerging data on the heterogeneity, and differential phenotypic and genotypic pattern of CTCs and DTCs as compared with the parental tumor cells, the observations on biological characteristics of such cells are immediately indicative of the clinical need to rethink current treatment decision algorithms and future study designs.
When Steven Paget published his theory of “seed and soil” in 1889,75 the idea of hematogenous tumor cell dissemination was born. More than a century later, with molecular tools being available, new clinical findings explained hitherto unexplainable phenomena, such as donor-derived cancer in recipient organ allografts76 or detection of viable single tumor cells in secondary organs being both descendants of a known primary tumor77 and potential precursors of subsequent metastasis.29 The fulfillment of the request for factors enabling individual risk assessment seems to be on the horizon. Yet, in breast cancer, recent guidelines for adjuvant systemic therapy still foresee treatment recommendations for more than 90% of patients, even in case of a negative lymph node status.78-80 The risk of tumor relapse in these patients is considered high enough to recommend adjuvant therapy, even though up to 70% of early-stage breast cancer patients are cured by locoregional surgery alone.
For their broad utilization, markers need to be implemented into current risk classification systems, such as the Tumor-Node-Metastasis (TNM) classification. Although the decision upon implementation appears to be pending since the 1990s, when DTCs were mentioned for the first time in the TNM Supplement,81,82 a useful proposal for an appropriate TNM terminology has recently been made by the International Union Against Cancer.83 The most recent TNM classification for breast cancer84 does not qualify the presence of single cancer cells in peripheral blood or bone marrow as metastasis (stage M0), but it optionally reports the presence of such cells together with their detection method (eg, M0[i+] or [mol+]) in analogy to its use for description of the presence of micrometastasis or isolated tumor cells in lymph nodes. CTCs and DTCs were only recently cited for the first time in the American Society of Clinical Oncology’s 2007 recommendations on the use of tumor markers.85
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree