Fig. 49.1
Breast cancer incidence and mortality in the world (GLOBOCAN 2012) (Reprinted from Breast Cancer. Estimated Incidence, Mortality Worldwide in 2012. Estimated age-standardised rates (World) per 100,000. From Ferlay et al. [138] Reprinted with permission from International Agency for Research on Cancer, World Health Organization)
MBC is diagnosed during the course of the disease in approximately 20–50% of women with early BC and in the majority of women originally diagnosed with locally advanced BC. Additionally 5–10% of women with newly diagnosed BC may present with metastatic disease (de novo MBC), again varying by geographic region/local screening practices/sociocultural and economic factors. BC metastasizes preferentially to the lungs, bones, liver, brain and distant lymph nodes. Different patterns of dissemination are observed in lobular BC, with frequent spread to the peritoneum and retroperitoneum, hollow viscera, internal genital organs and leptomeninges [1]. Patients frequently develop metastases at multiple sites.
MBC is incurable, which means that all patients will succumb to their disease eventually. Progress seen over the last few decades has led to significant improvements in the duration of survival, with recent series demonstrating median survival rates of between 2 and more than 5 years, depending on tumour phenotype and metastatic burden and site [2].
Most patients are diagnosed on examinations performed because of symptoms suggestive of metastatic disease; however, advances in the sensitivity and availability of imaging often lead to the diagnosis of metastatic disease earlier than in the past. This may be one of the factors contributing to the increased duration of post-MBC diagnosis survival; however, the scale of this phenomenon is unknown. The most commonly used imaging modalities include computed tomography (CT) for the chest (plain X-ray is acceptable for lesions surrounded by aerated lung), CT, magnetic resonance imaging (MRI) or ultrasonography for the abdomen, CT or MRI for the brain and radioisotope bone scan for the skeleton (often supplemented by CT or MRI in cases of diagnostic uncertainty). Positron emission tomography (PET) (usually merged with CT) is the most sensitive imaging modality; its drawbacks, however, include a relatively high false-positive rate and high cost. At diagnosis of MBC, patients should undergo imaging of the chest, abdomen and bone. In asymptomatic patients, brain imaging is unnecessary, irrespective of the phenotype of the tumour. Further tests should be performed in cases with symptoms suggesting involvement of sites not included in routine imaging. Additionally, routine haematology and biochemistry, in particular liver and renal function and serum calcium, should be performed to assess organ function and the feasibility of planned systemic therapy.
In most cases, imaging is conclusive for the diagnosis of MBC; however, in cases of doubt regarding the character of the lesion identified on imaging, a biopsy confirming its malignant character is obligatory before establishing the final diagnosis. Biopsy of the metastatic lesion is also increasingly recommended to confirm the biomarker status of the metastatic tumour as tumour phenotype may change. Some sources recommend metastatic biopsy at least once in all patients (if feasible); others believe that biopsy is not mandated if its results are not going to influence treatment choice [3, 4] – examples being a patient relapsing with bone-/soft tissue-only disease many years after diagnosis of a luminal breast cancer (who should be given a trial of endocrine therapy (ET)) or a HER2-positive patient relapsing with massive visceral disease (who should be given chemotherapy (ChT) with anti-HER2 treatment).
Treatment of MBC requires regular monitoring for toxicity and efficacy. Response assessment with clinical examination, lab evaluation and radiographic imaging should be performed on a regular basis (imaging every 2–3 cycles for ChT and every 2–4 months for ET) to enable timely termination of ineffective therapies and avoid exposing patients to unnecessary treatment and its toxicity. Lower imaging frequency is acceptable in patients with symptomatic improvement, long-standing remissions or disease stabilization. The same imaging modality should be used for all assessments, and a validated response assessment system, such as RECIST (Response Evaluation Criteria in Solid Tumours), should be used [5]. Because of good reproducibility, preferred imaging modalities are CT and MRI; ultrasound and radioisotope studies are not recommended due to lack of reproducibility or standardization. Circulating (serum) tumour markers, such as CA15-3 and CEA, are not recommended for routine use but may be useful as a supplementary tool in cases with difficult to assess disease, such as bone involvement only. Treatment decisions, however, should not be based solely on the degree of change of circulating tumour markers. Circulating tumour cells (CTCs), cell-free DNA and chemotherapy sensitivity and resistance assays (CSRAs) are experimental and should not be routinely used for treatment decision-making.
The prognosis in MBC depends predominantly on the tumour phenotype, with triple-negative BC carrying the worst prognosis, with a median survival of approximately 2 years and luminal tumours (including luminal HER2 positive) having the best outcomes, with median survivals in modern series extending to over 5 years. Other important prognostic factors include the patient’s performance status, the site and volume of metastatic disease, the duration of the disease-free interval and the history of previous anticancer treatments, including their efficacy.
MBC should be managed by a multidisciplinary team of all appropriate specialties (medical, radiation, surgical and imaging oncologists, palliative care, psychosocial and physiotherapy, among others) – preferably within the setting of a specialized Breast Unit. Treatment decision-making should include open discussion with the patients and their next of kin and setting realistic treatment goals. From the first diagnosis of MBC, patients should be offered personalized appropriate psychosocial, supportive and symptom-related interventions as a routine part of their care [6]. Treatment choice should take into account endocrine responsiveness, HER2 status, menopausal status, disease-free interval, previous therapies and response obtained, tumour burden (defined as number and site of metastases), biological age and comorbidities (including organ dysfunctions), performance status, need for rapid disease/symptom control, socio-economic and psychological factors, patient’s preferences and available therapies in the patient’s country [6]. As the aim of treatment is palliation and the realistic treatment goals include prolongation of life and improvement or preservation of its quality, but not cure, the least aggressive treatment modalities sufficient to achieve disease control should be used. This would usually mean ET for luminal breast cancer and single-agent ChT in hormone-nonresponsive disease. In HER2-positive disease, the «backbone» therapy should be combined with anti-HER2 agent(s).
Treatment for metastatic disease is complex and multimodal and may require combinations of systemic and/or regionally ablative/palliative therapies for optimal care. The latter are discussed generally in this chapter and covered in more details in ► Chaps. 56 (lung), 53(bone), 55(hepatic) and 54 (brain).
Systemic treatment will be discussed separately for each BC phenotype – ◘ Fig. 49.2. For definition of the phenotypes, please refer to ► Chap. 15.
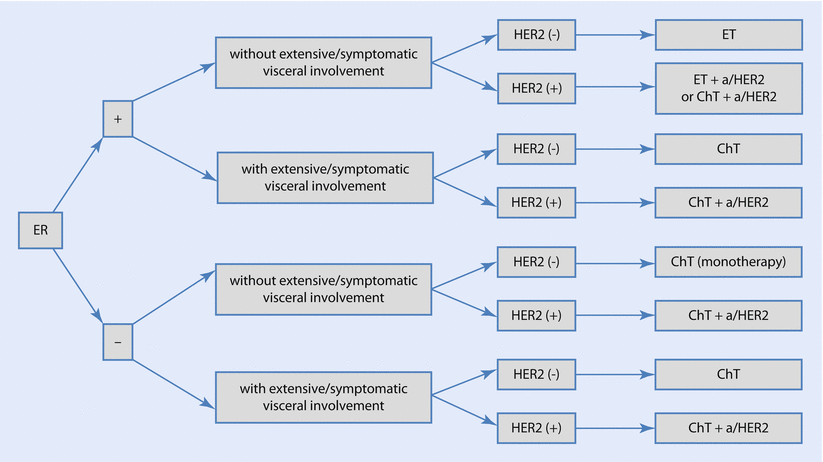

Fig. 49.2
Strategy of systemic treatment of MBC. a/HER2 anti-HER2 therapy, ChT chemotherapy, ER oestrogen receptor, ET endocrine therapy, HER2 human epidermal growth factor receptor 2, T trastuzumab (Reprinted from F. Cardoso et al. [139], ◘ Fig. 49.2. With permission from Oxford University Press on behalf of the European Society for Medical Oncology ©)
49.2 Luminal, HER2-Negative BC
Luminal HER2-negative (ER/PgR+/HER2-) BC in 2010 in the USA accounted for 61.2% of primary stage IV BC and, as it constitutes almost three quarters of all newly diagnosed BC, remains also the most prevalent subtype among patients who relapse following treatment for early disease [7].
The systemic treatment of choice in luminal BC is ET; it should be considered in all patients, unless contraindications exist, as its use is associated with better health-related quality of life (QoL), greater satisfaction with treatment, less treatment-related side effects and less activity impairment [8]. Contraindications to ET include massive visceral involvement/directly life-threatening disease (visceral crisis) and proven resistance to ET. Unfortunately data supporting the informed choice between ET and ChT is rather limited and comes mainly from non-randomized comparisons. All available randomized studies were conducted in the 1970s and 1980s and compared often obsolete ChT schedules and suboptimal endocrine agents. A meta-analysis of these studies demonstrated, however, no effect of the treatment strategy on overall survival (OS) in spite of a higher response rate (RR) in the ChT group [9]. No randomized comparisons are available between aromatase inhibitors (AI) or fulvestrant and modern chemotherapeutic agents, such as taxanes, capecitabine, vinorelbine or eribulin. Lack of high level of evidence supporting treatment choices in metastatic luminal BC forces oncologists to rely on indirect evidence from retrospective studies or prospective non-comparative data. Although fair comparisons of results in patients treated with ChT and ET outside studies randomizing subjects between these two options are impossible, in general, those treated with first-line ET achieve longer progression-free survival (PFS) and OS. Obviously, populations selected for ChT and ET are different, but these differences are smaller than could be expected: in general, the percentage of ER/PgR-positive patients in ChT studies ranges between 70% and 80%, whereas visceral involvement is present in about 50–80% of patients undergoing ChT and in about 50% of those treated with ET [10].
The main ET options include selective oestrogen receptor modulators (SERM, tamoxifen), selective oestrogen receptor downregulators (SERD, fulvestrant) and AIs. Premenopausal patients should additionally undergo oophorectomy or medical castration by use of luteinizing hormone-releasing hormone (LHRH) agonists. In selected cases with a good response to previous lines of ET, more toxic compounds such as progestins, oestrogens or androgens can also be considered. Available clinical data don’t point to obvious superiority of any of the available ET compounds. Some of first-line studies comparing tamoxifen with AI have reported improvements in response rates (RR) and time to progression (TTP) for AIs. In the second-line setting, AIs have consistently demonstrated improved efficacy and decreased toxicity in comparison to the first-generation AI aminoglutethimide and megestrol acetate [11]. Additionally, a minor OS benefit (HR 0.9) of AI vs. non-AI treatment was seen in the Cochrane review of available studies [11]. Early studies with low-dose (250 mg every 4 weeks) fulvestrant showed no additional benefit from this agent [12]; more promising are the data for high-dose fulvestrant (500 mg 4 weekly + an additional dose after the first 2 weeks). A greater degree of efficacy, both in terms of TTP and OS, of high-dose fulvestrant was first demonstrated in the CONFIRM study comparing it with the originally approved dose of 250 mg [13]. Particularly, promising are the data from the phase II FIRST study comparing high-dose fulvestrant to anastrozole in first-line treatment, which demonstrated significant and clinically relevant OS prolongation (HR 0.7) [14], and results of the confirmatory phase III FALCON study have recently been released demonstrating significant PFS prolongation [15]. In a single study (SWOG S0226), the combined use of fulvestrant (low dose) and an AI vs. an AI alone resulted in significant PFS and OS prolongation, with the largest benefit in patients not exposed to prior tamoxifen and in those with the longest DFI [16]; this was not, however, confirmed in the subsequent study (FACT) and in the meta-analysis of both [17, 18].
In premenopausal patients, the only compound with demonstrated activity as monotherapy is tamoxifen; however, available randomized trials demonstrated RR, PFS and OS improvement from the addition of a LHRH agonist to tamoxifen, so combination treatment is recommended [19]. AI are inactive as single agents in premenopausal patients and should always be combined with ovarian ablation or suppression. Caution is needed to assure «postmenopausal» oestrogen levels in AI-treated patients, as in approximately 40% of those who developed amenorrhoea as a result of ChT and in 17–25% of patients on an AI plus LHRH agonist therapeutically effective levels of oestrogen suppression may not be present [20, 21]. Ovarian suppression or ablation should be continued throughout the course of ET [22]. No data are available on the efficacy of fulvestrant as a single agent in premenopausal MBC women, although its mechanism of action and a single preoperative study suggests it may actually be effective [23].
Particular ET compounds differ in their toxicity profiles: AI use is related to an increased risk of arthralgia, myalgia, tendonitis and carpal tunnel syndrome, vaginal dryness and decreased libido, hot flashes, bone mineral loss and fractures, and tamoxifen use increases the risk of menopausal symptoms including hot flashes and gynaecologic complications as well as endometrial hyperplasia and cancer, and thromboembolic events [24, 25]. Fulvestrant in comparison to «other endocrine therapy» (mostly AI) demonstrated a similar toxicity profile, except for a lower incidence of arthralgia [12].
The general philosophy of systemic therapy for metastatic luminal breast cancer is to continue treatment with endocrine agents for as long as the patient is deriving benefit [3, 4, 26] which means that patients progressing following effective first-line ET should be offered next ET lines, and switching to ChT should be undertaken only in those with proven resistance to endocrine manoeuvres or with rapidly progressive visceral disease (◘ Fig. 49.3). The optimal ET sequence is unknown, and treatment choice depends on menopausal status, prior ET, response duration, drug toxicity profile and availability and the patient’s preferences.
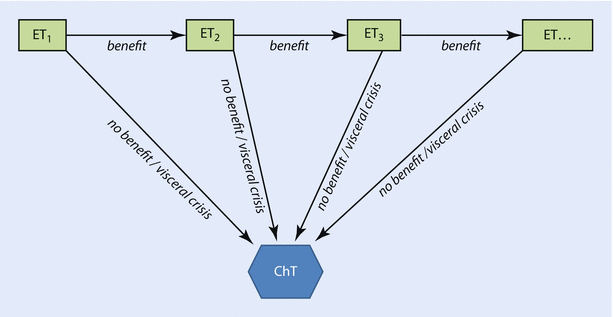

Fig. 49.3
Strategy of systemic treatment of luminal BC. ChT, chemotherapy; ET, endocrine therapy trastuzumab
Importantly, among ET-treated luminal MBC, the tumour response is not a surrogate for long-term benefit, and similar OS is observed in those achieving objective tumour regression or long-term disease stabilization [27]. It thus needs to be kept in mind that, as most patients undergoing early lines of treatment for MBC are asymptomatic or mildly symptomatic and do not require rapid symptomatic improvement, the aim of the treatment in the majority of cases is delaying disease progression (prolonging PFS) and not necessarily obtaining tumour shrinkage.
Additionally, not all BCs are equally sensitive to ChT, and in some patients, ET may actually be more effective. As demonstrated in neoadjuvant studies, the frequency of complete pathological remissions following ChT varies significantly between BC phenotypes, being the lowest for ER/PgR+/HER2- patients, in particular for lobular cancers [28–30]. Additionally, some of the effect of ChT in oestrogen receptor (ER)-positive premenopausal patients may not necessary come from its cytotoxic-antineoplastic activity. As demonstrated in the adjuvant NSABP B-30 and IBCSG 13–93 studies, long-term outcomes are superior in patients who become amenorrhoeic as a result of ChT [31, 32], so it can be hypothesized that at least some of the therapeutic effect in this population is actually effected by the endocrine mechanism of action and could be more simply obtained directly with ET.
ET is also feasible in patients with visceral involvement, as long as there is no directly life-threatening disease (visceral crisis) [3, 4, 26]. Indeed, as demonstrated in data from 1396 patients from four phase III studies of first-line ET, the response rate is higher in non-visceral metastases, but if disease control is achieved, its duration is equal in patient with and without visceral involvement [33].
Unfortunately, no biomarkers predictive of ET benefit beyond ER and PgR have been identified for luminal HER2-negative BC. Some help may be provided by analysis of clinical factors, although results are often inconsistent. Various studies suggest the largest benefit from ET is seen in patients with lower tumour grade; longer disease-free interval; lack of liver, CNS or multiple-site involvement; no history of adjuvant ET; strong expression of ER and PgR; a history of clinical benefit from first-line ET; and a lower tumour proliferation rate (identified by Ki67 expression) [34–39]. A number of novel molecular factors potentially predictive of endocrine responsiveness have been described, but none has been properly validated. Recently, the prognostic role of the 21-gene recurrence score (Oncotype DX) for both TTP and 2-year OS in ER/PgR+/HER2- patients was demonstrated in a prospective series of de novo stage IV BC [40].
ET (and indeed any other therapy) in MBC has limited activity, and all patients inevitably develop progressive disease. This endocrine resistance in MBC, for the purpose of patient stratification for clinical research, has been somewhat artificially divided into primary resistance, defined as progressive disease (PD) within the first 6 months of first-line ET for MBC, while on ET and secondary resistance, i.e. PD developing 6 or more months after initiating ET for MBC, while on ET [3, 4]. There are various mechanisms suggested as responsible for endocrine resistance development, including ER loss, ER mutation or activation of alternative signaling pathways.
Loss of the oestrogen receptor may be caused by epigenetic changes to the ESR1 gene coding for the ER (methylation, histone modifications), and studies are ongoing testing histone deacetylase inhibitors (HDACi, which inhibit a key means of epigenetic regulation of gene expression) in combination with ET. A phase II ENCORE 201 study evaluating the combination of exemestane and HDACi entinostat demonstrated OS prolongation in patients progressing on a non-steroidal AI, and the compound was granted «breakthrough therapy designation» by the FDA; confirmatory phase III study results are awaited [41].
Genomic alterations in the ER, very rare in early breast cancer, are observed with increasing frequency in advanced disease, reaching >30% in late MBC [42, 43]. They usually include gain of function point mutations in the ligand-binding domain of the ER protein leading to its constitutive (ligand-independent) activity [44].
Development of a malignant phenotype is associated with dysregulation of numerous cell signaling pathways. Those implicated in the development of endocrine resistance and potentially pharmacologically «targetable» include activation of tyrosine kinase growth factor receptors, PI3K-Akt-mTOR pathway and dysregulation of the cell cycle [45].
Targeting growth factor receptors has provided the most valuable clinical data for HER2-positive disease; this is further discussed in the «Luminal B HER2-Positive Breast Cancer» section. Studies evaluating the role of blocking other growth factor receptors (EGFR, HER3, IGF, FGFR, MET) in combination with ET have so far not been successful [45].
Alterations of the PI3K/AKT/mTOR pathway are among the most frequent genomic abnormalities present in luminal BC [46]. In ER-positive breast cancer cell lines and tumour models, PI3K pathway activation confers endocrine resistance via crosstalk between ER and the receptor tyrosine kinases activating the pathway, as well as through ligand-independent ER activation through mTORC1 [47].
The first approved treatment targeting the PI3K/AKT/mTOR pathway in combination with ET was the mTOR inhibitor everolimus combined with exemestane. In the randomized phase III BOLERO-2 study, clinically significant PFS prolongation has been demonstrated for this combination in patients progressing on or after treatment with a non-steroidal AI; unfortunately this has not translated into an OS benefit, and the treatment was associated with significant toxicities, often leading to treatment interruptions and dose reductions, occasionally requiring permanent drug discontinuation [48, 49]. Phase II experience with PI3K inhibitors both in combination with ET and ChT has so far been disappointing, at least partially due to toxicity limiting optimal dosing [50, 51]. In the phase III BELLE-2 study, PFS prolongation was seen in patients treated with a combination of a pan-PI3K inhibitor buparlisib and fulvestrant, with the largest benefit observed in patients with PIK3CA mutations present in circulating tumour DNA, possibly providing a predictive factor for this therapy [52]. Unfortunately, significant toxicity (transaminase rise, rash, hyperglycaemia and mood disorders) led to frequent treatment discontinuations, potentially limiting treatment applicability.
Other attractive targets are cyclin-dependent kinases (CDK) which control the cell cycle. Combination of the CDK4/CDK6 inhibitor palbociclib and ET in patients with advanced ER/PgR+/HER2- BC resulted in a significant PFS prolongation in three randomized trials either in combination with an aromatase inhibitor in the first-line setting (PALOMA-1, PALOMA-2) or with fulvestrant in patients who had relapsed or progressed during prior endocrine therapy (PALOMA-3) [53, 54]. This has led to accelerated FDA approval of this compound in patients with ER-positive advanced BC as initial endocrine-based therapy for metastatic disease and recently also for second-line therapy. Unfortunately, no OS benefit has been so far observed [53]. Recently, positive results of a phase III MONALEESA-2 study of letrozole ± ribociclib and MONARCH 2 study (of fulvestrant +/– abemaciclib) (another CDK4/CDK6 inhibitor) were also announced, and drug approval [55, 56]. The advantage of agents in this class is their favourable toxicity profile.
As an interaction exists between endocrine regulation and angiogenesis mediated by VEGF, and higher levels of VEGF are associated with a decreased response to ET [57, 58], and the combination of anti-VEGF treatment with ET could potentially increase the efficacy of ET. To test this hypothesis, bevacizumab (a monoclonal antibody against VEGF) was evaluated in combination with endocrine treatment in the LEA and CALGB 40503 trials. Although the CALGB 40503 trial demonstrated a PFS improvement for the combination of letrozole and bevacizumab, neither of the studies demonstrated an OS benefit, and the combined treatment was associated with increased toxicity [59, 60].
In spite of the endocrine responsiveness and the preference for ET, all patients at some stage require cytotoxic ChT. Its principles, however, do not differ from those in other BC subtypes and will be discussed in the «triple-negative BC» section.
49.3 HER2-Positive Breast Cancer
Human epidermal growth factor receptor type 2 (HER2) is overexpressed in approximately 15 to 20% of invasive breast cancers and was historically associated with a poor prognosis [61]. The development of HER2-directed therapies in the past 15 years has changed the natural course of the disease and offers patients with HER2-positive BC survival at least equivalent to patients without HER2 alteration. Currently, median OS in metastatic HER2-positive BC exceeds 50 months, which compares favourably with survival of approximately 20 months observed before the anti-HER2 treatment era [2]. Approved HER2-directed agents include trastuzumab (human monoclonal IgG antibody that selectively targets HER2), pertuzumab (monoclonal antibody that blocks the formation of HER2:HER3 heterodimers), trastuzumab emtansine (conjugate of trastuzumab and cytotoxic emtansine – T-DM1) and lapatinib (an oral tyrosine kinase inhibitor that functions downstream of HER2, inhibiting both EGFR and HER2 receptors).
There are several rules guiding the treatment of HER2-positive metastatic disease:
HER2-directed therapy should be initiated early in the course of the disease.
Anti-HER2 agents can be partnered with ChT or ET and can also be used in combination (dual HER2 inhibition).
In women progressing on an anti-HER2 therapy, continuation of HER2-directed therapy with alternative or the same (only for trastuzumab) agent is recommended since continued suppression of the HER2 pathway improves outcomes.
With the exception of trastuzumab, no data support continuing treatment with the same agent beyond progression.
Due to the risk of cardiac toxicity associated predominantly with trastuzumab, cardiac assessment is recommended before initiation of treatment and regularly during therapy. Serious cardiac comorbidities and/or a left ventricular ejection fraction of less than 50% are contraindication to the treatment. In addition, trastuzumab should not be administered concurrently with anthracyclines.
HER2-positive BC represents two molecular subtypes: HER2-positive non-luminal and HER2-positive luminal BC (co-expressing HER2 and ER/PgR). In both groups, anti-HER2 agents are important components of therapy; however, there are substantial differences regarding tumour biology and treatment choices.
Despite the breadth of trials with HER2-directed agents, a number of questions remain regarding the optimal use of anti-HER2-targeted therapy in MBC such as treatment sequence, regimens, duration of the ChT component and the combination with ET in luminal HER2-positive tumours.
49.3.1 HER2-Positive, Non-luminal Breast Cancer
Trastuzumab was the first targeted agent studied and subsequently approved for the treatment of advanced HER2-positive BC. In the pivotal trial, trastuzumab added to standard ChT improved time to progression (median TTP, 7.4 vs. 4.6 months; p < .001) and increased median OS from 20.3 to 25.1 months (p = .046) [62]. There are a number of cytotoxic agents which can be partnered with trastuzumab, but the preferred first-line agents for use in combination are paclitaxel, docetaxel, vinorelbine and capecitabine. The optimal duration of ChT in combination with trastuzumab is not known. Based on the design of clinical trials, current guidelines recommend continuance of ChT for 4–6 months or longer in the absence of disease progression and relevant toxicities. Duration of ChT should depend on toxicity and response. If ChT is stopped due to adverse effects or maximal response, anti-HER2 therapy alone should be continued. Currently, there is no evidence to support treatment breaks in patients who have a durable response.
Pertuzumab was incorporated into standard combinations of trastuzumab and ChT, because it had been hypothesized that blocking the ability of HER2 to heterodimerize with other members of the HER family, inhibits HER2 signaling by preventing ligand-activated HER2/HER3 or HER2/HER1 heterodimerization and may partially overcome resistance to trastuzumab. In the CLEOPATRA trial, the combination of trastuzumab, docetaxel and pertuzumab outperformed trastuzumab with docetaxel, with an increase in median PFS of 6 months (18.6 vs. 12.4 months; p < .001) and an increased median OS of more than 15 months (56.5 vs. 40.8 months; p = .002) [63]. Currently double HER2 blockade with trastuzumab and pertuzumab combined with docetaxel is the preferred first-line regimen. With the lack of predictive biomarkers other that HER2, it is not known, however, whether all patients with HER2-positive BC derive more benefit from dual HER2 blockade, compared to trastuzumab plus ChT [64]. There is no further evidence of superiority of pertuzumab-containing regimens in first-line treatment. In the MARIANNE study, addition of pertuzumab to T-DM1 provided no efficacy benefit compared to trastuzumab and taxane or T-DM1 alone in this setting [65].
If pertuzumab is not available, trastuzumab with ChT remains a valid first-line treatment option. Trastuzumab-containing regimens are superior to those with lapatinib. Two trials (MA31 and CEREBEL) comparing trastuzumab and ChT vs. lapatinib and ChT in the first and further lines setting demonstrated lower PFS and more adverse events in the lapatinib arms [66, 67].
In the second-line setting, several regimens are effective. In the EMILIA trial comparing T-DM1 versus lapatinib plus capecitabine in patients who had previously received trastuzumab and a taxane, a significant increase in median PFS of 3.2 months (9.6 vs. 6.4 months, HR 0.65; p < .001) and median OS of 5.8 months (30.9 vs. 25.1 months; HR 0.68; p < .001) in favour of T-DM1 was shown [68]. In addition, T-DM1 was better tolerated than the lapatinib plus capecitabine and is recommended as the standard second-line treatment. Another option is continuation of trastuzumab with a different cytotoxic agent. In the German GBG-26 trial of trastuzumab and capecitabine vs. capecitabine alone, combined treatment resulted in a superior TTP and a non-significant OS benefit; the study was, however, underpowered due to poor recruitment [69]. Similarly, continuing trastuzumab with lapatinib beyond progression on a trastuzumab-based regimen outperformed lapatinib alone in both PFS and OS [70]. Lapatinib added to capecitabine in patients previously treated with an anthracycline, a taxane and trastuzumab resulted in an improvement in median TTP (8.4 vs. 4.4; HR 0.49; p < .001), without significant OS benefit, compared to capecitabine alone [71, 72]. The use of pertuzumab beyond the first line is not recommended. In the PHEREXA study, addition of pertuzumab to trastuzumab and capecitabine in the second-line setting did not significantly improve PFS but increased median OS by 8 months; this was not, however, statistically significant [73].
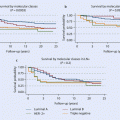
Patients who have not received T-DM1 in the second-line setting may still benefit from T-DM1 beyond the second line. The TH3RESA trial performed in patients who have had prior therapy with a taxane, trastuzumab and lapatinib, comparing T-DM1 with treatment of physician’s choice, demonstrated doubling of PFS and an OS benefit of about 7 months in favour of T-DM1 [74] (◘ Fig. 49.4).
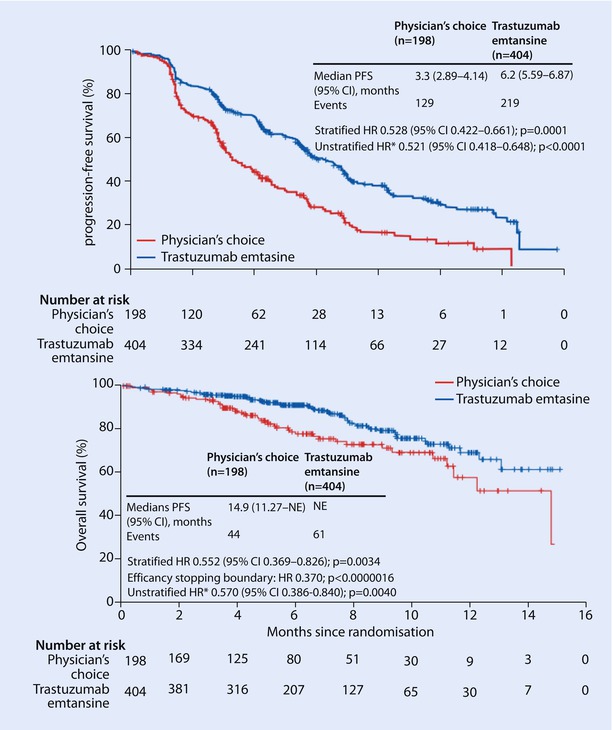

Fig. 49.4
TH3RESA study: Kaplan-Meier curves of progression-free survival and overall survival (From Krop et al.[74]. Reprinted with permission from Elsevier)
Because most first-line anti-HER2-therapy trials were performed in treatment-naive or predominantly anti-HER2 treatment-naive populations, management of patients who have relapsed after adjuvant trastuzumab is not well determined. Women with a disease-free interval (DFS) of more than 12 months since prior neoadjuvant/adjuvant treatment with trastuzumab may be offered pertuzumab with trastuzumab and docetaxel, as such a group was enrolled in the CLEOPATRA trial. Of note, 90% of women in this trial had not received prior trastuzumab therapy. Due to the small numbers and large confidence intervals, it’s not clear whether women who were previously exposed to trastuzumab derive comparable benefit from pertuzumab as those who are trastuzumab naive. Despite improvement demonstrated in the subgroup analysis in patients who relapsed after adjuvant trastuzumab, the outcome in this population was inferior compared to the trastuzumab-naive group (OS 46.6 months vs. 53.8 months, respectively; HR 0.8; 95% CI, 0.44–1.47) [63]. If pertuzumab is not available, trastuzumab with ChT is the recommended regimen. Data from two studies in the first-line setting, including patients diagnosed with de novo HER2-positive MBC and those who have relapsed after adjuvant therapy, suggest superiority of retreatment with trastuzumab and ChT over lapatinib and ChT [66, 67]. Women with early relapses (DFS of less than 6 months) indicating resistance to trastuzumab are candidates for second-line therapy, preferably T-DM1. There is a paucity of data regarding treatment in women with a DFS of more than 6 months and less than 12 months and both first- and second-line options can be considered.
The use of dual anti-HER2 blockade without a ChT backbone in metastatic HER2-positive BC is not supported by high level of evidence. Combination of lapatinib plus trastuzumab compared with lapatinib alone in patients with trastuzumab refractory disease improved PFS and OS; however, lapatinib alone seems to be a suboptimal comparator [70]. In the MARIANNE study, dual blockade with pertuzumab and T-DM1 was not superior to trastuzumab combined with a taxane, although the combined targeted therapy was associated with a much more favourable toxicity profile [65]. Despite the activity of ChT-free anti-HER2 therapies, due to lack of biomarkers which identify the subgroup of patients for whom the combination of anti-HER2 agents alone may be sufficient, such an approach cannot be recommended as standard.
49.3.2 Luminal B HER2-Positive Breast Cancer
Luminal HER2-positive tumours represent approximately half of all HER2-positive BC. ER/PgR and HER2 status are the key factors determining a patients’ prognosis and response to therapy. Tumours co-expressing HER2 and ER/PgR are less responsive to ET than luminal HER2-negative tumours. On the other hand, multiple studies have demonstrated that benefits from HER2-directed agents depend on ER/PgR status and HER2 inhibition is less effective in ER-positive BC compared to ER-negative tumours [75, 76].
The importance of concurrent ER and HER2 blockade in the treatment of this subset of patients is well recognized, and the concept of combined endocrine and anti-HER2 therapies is supported by preclinical and clinical data, but the optimal use of ET and anti-HER2 agents has not been determined.
The majority of patients with luminal HER2-positive tumours are offered HER2-directed therapy combined with ChT, because the benefits from ChT-containing regimens were demonstrated in both luminal and non-luminal HER2-positive subtypes across all studies, with improvements in response rates, PFS, TTP and OS. Data regarding ET use in luminal HER2-positive patients is limited to three relatively small trials, which showed that HER2-targeted therapy added to ET improved the response rate and PFS, but the impact on OS was not formally assessed. In the TAnDEM (Trastuzumab and Anastrozole Directed Against ER-Positive HER2-Positive Mammary Carcinoma) trial comparing anastrozole plus trastuzumab with anastrozole alone, median PFS was doubled in the combination group (4.8 vs. 2.4 months; HR 0.63 95% CI, 0.47 to 0.84; p = .0016) [77]. Despite substantial crossover (70% of patients) at disease progression, a numerical difference in median OS of 4.6 months favouring trastuzumab and anastrozole (28.5 vs. 23.9 months; log-rank p = .325) was observed. Similar results were achieved in the EGF100151 study of lapatinib plus letrozole versus letrozole, performed both in HER2-positive and HER2-negative BC patients [78]. Significant improvement in PFS (median PFS of 8.2 vs. 3.0 months; HR 0.71; 95% CI, 0.53 to 0.96; p = .019) in the HER2-positive group and increased overall RR (28% v 15%; p = .021) translated into a non-significant OS difference (median OS of 33.3 vs. 32.3 months; HR = 0.74; 95% CI, 0.5 to 1.1; p = .113). The third study – eLEcTRA (Study of the Efficacy and Safety of LEtrozole Combined with TRAstuzumab) – was closed prematurely due to poor accrual, and analysis restricted to 92 patients showed non-significant improvement in TTP [79]. Importantly, combination of ET plus anti-HER2 therapy was associated with minimal adverse effects.
Overall, the improvement in PFS across trials with ET and HER2-directed therapy was modest, and although direct comparison of ET plus HER2-targeted therapy with ChT plus HER2-targeted therapy has never been performed, indirect data suggest better outcome in patients who received ChT with an anti-HER2 agent. However, given the significant PFS benefit and favourable toxicity profile of ET combined with anti-HER2 therapy, a subset of luminal HER2-positive patients with asymptomatic/mildly symptomatic, indolent, low-volume disease, a long disease-free interval or contraindications to ChT may be candidates to such treatment.
ET may also be used in combination with HER2-targeted agent as a maintenance regimen in patients who initially received ChT combined with anti-HER2 therapy, and the cytotoxic component was stopped at the point of maximal response and/or toxicity. Such an approach, not supported by high level of evidence, is widely used in practice, as it offers effective and minimally toxic treatment. Data from observational studies such as RegistHER favours sequential use of ChT and ET over concurrent use with regard to both PFS and OS (adjusted PFS HR (0.81), 95% CI (0.54–1.21); adjusted OS HR (0.48), 95% CI (0.26–0.89)) [80].
49.4 Triple-Negative Breast Cancer
Metastatic triple-negative breast cancer (TNBC) compared to other BC subtypes is characterized by aggressive biology, significantly shorter disease-free and overall survival times and a tendency toward visceral (vs. bone) metastases. Patients with early-stage TNBC have a high risk for early relapse. The pattern of relapse in TNBC is distinct, with a rapidly rising rate in the first 2 years following diagnosis, reaching a peak at 2 to 3 years and a decline in recurrence risk over the next 5 years [81]. Unlike other subtypes, no targeted therapy has proven efficacy in the treatment of TNBC; thus, chemotherapy (ChT) remains a mainstay of systemic treatment. ChT may be used also in the treatment of other BC subtypes, i.e. in endocrine-resistant luminal BC or in patients with rapid progression, symptomatic or high-volume visceral disease, in whom ET is unlikely to result in prompt response and clinical improvement. Most of the general principles regarding ChT discussed in this chapter apply also to advanced breast cancers of other phenotypes requiring ChT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree