Metastatic Breast Cancer
INTRODUCTION
Since 1990, the annual rate of breast cancer death has been decreasing by approximately 2.2% per year (1). Historically, median survival of patients with metastatic breast cancer (MBC) was estimated to be 18–30 months. Many experts agree that median survival has improved in recent years beyond 30 months, although survival varies significantly by breast cancer subtype. A number of newer active agents have recently been added to the armamentarium against breast cancer, including third-generation aromatase inhibitors, novel antimicrotubule chemotherapy agents, and biologic agents such as lapatinib, pertuzumab, and everolimus. Despite these advances, breast cancer remains the second leading cause of cancer death in women in the United States, with 39,620 women estimated to die of breast cancer in 2013 (1).
 PROGNOSIS
PROGNOSIS
Several factors contribute to predicting an individual patient’s course of disease:
• Prolonged relapse-free survival of more than 5 years is more favorable.
• Isolated chest wall or ipsilateral nodal recurrence predicts better outcome than visceral disease.
• Bone and soft-tissue recurrence is more favorable than visceral or central nervous system disease.
• The prognosis of HER2-positive MBC is not established but median survival may be well beyond 3 years with the advent of newer HER2-directed strategies (2, 3).
• The prognosis of triple negative breast cancer (ER/PR/HER2 negative) remains poor with median survival of approximately 1 year (4).
• A prognostic index predicts median overall survival of 50, 23, and 11 months for low-, intermediate-, and high-risk groups, respectively (Figure 59-1) (5). However, its utility is limited in clinical practice because it does not account for differences in breast cancer subtypes that impact treatment options.
• Up to 2%–3% of patients with favorable characteristics may be long-term survivors with over 20-year survival. Such patients tend to be young, have limited disease, and have a complete response to initial therapy.
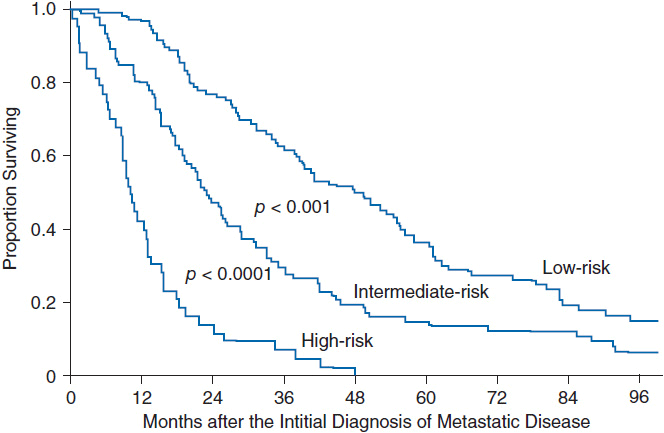
FIGURE 59-1 Prognostic index for patients with metastatic breast cancer. Patients with MBC were stratified into risk groups based on the total sum of individual prognostic factors as follows: (1) adjuvant chemotherapy—add 1 point if received; (2) distant lymph node metastates—add 1 point if present; (3) liver metastases—add 1 point if present; (4) lactate dehydrogenase—add 1 point if >1 × normal; (5) disease-free interval—add 2 points if <24 months. Low-risk group ≥1 point, intermediate group = 2–3 points, high risk ≤4 points. (Adapted from Reference 5 with permission.)
 GOALS OF TREATMENT
GOALS OF TREATMENT
Metastatic breast cancer is not considered curable. Therefore, the goals of treatment must carefully balance the risks of treatment-induced toxicity with the expected clinical benefit. Accepted clinical endpoints in the treatment of MBC include prolonged overall survival, improved quality of life (QOL), progression-free survival (PFS), and cancer-related symptom control.
DIAGNOSTIC EVALUATION
Most patients with MBC present with a recurrence following treatment for early-stage breast cancer. Less than 10% of patients present with MBC at the time of initial diagnosis. The most common sites of metastatic recurrence are bone, lungs, liver, and the central nervous system. Up to 50%–75% of patients will have single organ involvement at the time of initial diagnosis with MBC. Local recurrence after mastectomy usually involves the chest wall or overlying skin. In such cases, distant metastatic disease is present in 25%–30% of cases.
Evaluation of patients presenting with recurrent or MBC includes the following (6):
• History and physical exam
• Complete blood count
• Liver function tests
• Chest CT
• Abdomen and pelvis CT (or MRI)
• Positron emission tomography (PET) scan (optional, but of low added value in most cases)
• Bone scan, with subsequent radiographs of areas of concern for fracture
• Biopsy to confirm first metastatic recurrence, with confirmation of hormone receptor and HER2 preferred
Biopsy-proven confirmation of the first metastatic recurrence is preferred. The presence of estrogen receptor (ER), progesterone receptor (PgR), and HER2 overexpression in tumors will influence the selection of therapy. Therefore, biopsy of initial metastatic recurrence is increasingly clinically indicated to confirm ER, PgR, and HER2 status. In addition, patients with a history of breast cancer are at increased risk for secondary non-breast cancers, which may affect treatment selection.
TREATMENT
 OVERVIEW
OVERVIEW
A treatment algorithm for MBC is shown in Figure 59-2. Although treatment options have improved significantly in recent years, most patients with MBC will eventually succumb to the disease and, therefore, all patients with MBC should be considered for participation in clinical trials.
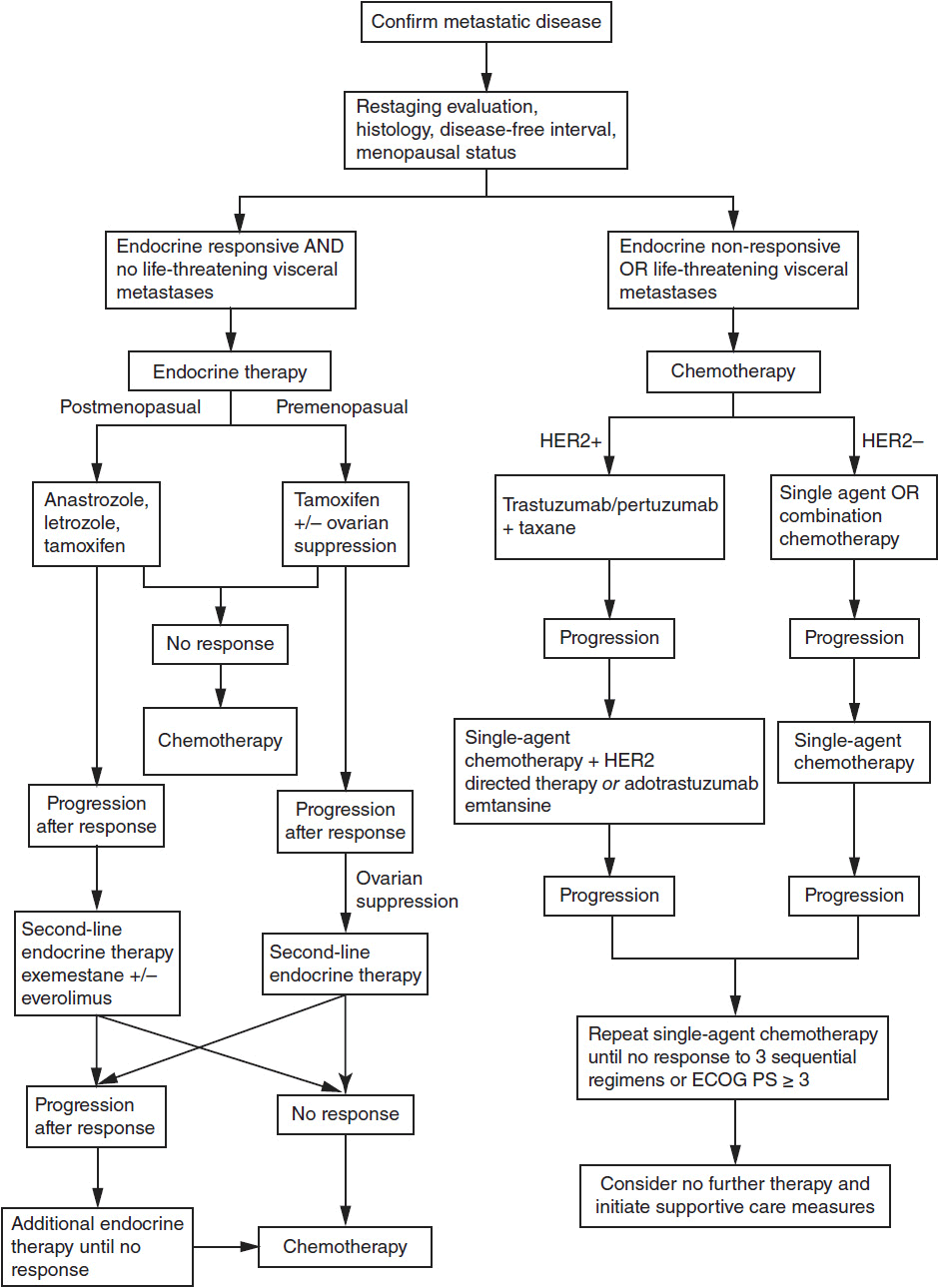
FIGURE 59-2 Treatment algorithm for metastatic breast cancer.
Selection of initial and subsequent treatment requires consideration of multiple factors:
• Patient goals
• Social support
• ER, PgR, and HER2 status
• Sites of disease
• Comorbid conditions
• Performance status (ECOG or Karnofsky)
• Toxicities of treatments
• Pace of disease progression
• Previous treatment and responses
• Need for concurrent localized therapy (e.g., radiotherapy or surgery for bone or CNS disease)
• Likelihood of response to treatment
 MONITORING TREATMENT
MONITORING TREATMENT
Treatment may be followed by periodic evaluation of the following:
• History and physical exam—Monthly evaluation for patients with MBC is reasonable to assess for progression and toxicities of treatment, although for patients on endocrine therapy less frequent follow-up may be appropriate.
• Serum tumor markers: CA15-3, CA27.29, and CEA—The serum markers CA15-3 and CA 27.29 correlate with the course of disease in 60%–70% of patients, and carcinoembryonic antigen (CEA) levels correlate in 40%. Elevated serum markers prior to treatment initiation allow periodic monitoring of marker levels during the course of treatment. A “flare” reaction often occurs with endocrine therapy causing a rise in serum markers in the first month of treatment. The only recommended use of serum tumor markers is to monitor patients with MBC, according to an expert panel convened by the American Society of Clinical Oncology (7). Although assessment of circulating tumor cells (CTCs) is available for patients with MBC, its utility in routine clinical care remains controversial and most expert panels do not recommend its use.
• Radiographic studies: CT, MRI, PET, bone scans, plain films—Standardized guidelines for radiographic monitoring of patients with MBC are not established and monitoring must be individualized for each patient. Periodic scans in the absence of rising tumor markers or changing symptoms are unlikely to affect survival. Radiographic evaluation is appropriate if symptoms change or tumor markers rise. CT scans are useful to establish a new baseline after maximum response to a given therapy is achieved. PET scans are currently approved for MBC for staging and monitoring response to therapy. However, its value in clinical decision making is not established. Routine use of MRI or CT scans to monitor the central nervous system is not recommended unless a patient has known CNS disease or presents with symptoms suggestive of CNS involvement.
 ENDOCRINE THERAPY
ENDOCRINE THERAPY
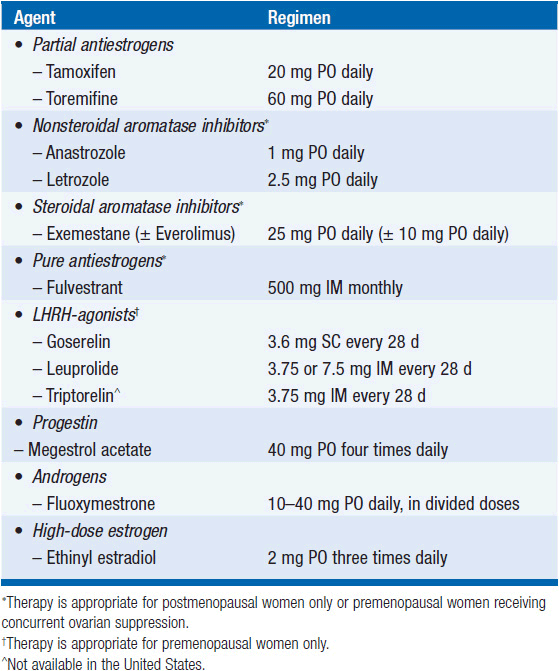
Endocrine therapy is preferred for most patients with hormone receptor positive MBC. Hormonal agents used in MBC are listed in Table 59-1. The mechanism of action and toxicities are described in more detail in Chapter 11, “Hormonal Agents: Antiestrogens.” The following general guidelines apply to selection of hormonal therapy for MBC (Figure 59-2):
• Endocrine therapy should be initial treatment for patients with hormone receptor-positive tumors without symptomatic visceral metastases or rapidly progressive disease.
• The response rates to hormonal therapy for ER+/PgR+, ER+/PgR-, ER-/PgR- are 70%, 40%, and 10%, respectively.
• Patients with primary endocrine resistance generally should proceed directly to chemotherapy.
• Patients who respond to initial endocrine therapy may proceed to additional lines of endocrine therapy until no further response is attained or visceral metastases develop requiring rapid and more effective response. The response rate to second-line endocrine therapy is up to 33% in patients who initially responded to tamoxifen and 15% in patients who had no initial response.
• Tamoxifen is commonly used as first-line hormonal therapy in both pre- and postmenopausal women with MBC. Response rates are as high as 50%–60% and may last on average over 12 months in patients without prior exposure to adjuvant endocrine therapy. A “tamoxifen flare” can result in bone pain and increased metastatic skin lesions in up to 13% of patients. Withdrawal of tamoxifen can induce a secondary withdrawal response. Anastrozole and letrozole are third-generation nonsteroidal aromatase inhibitors (AIs) approved for first-line use in postmenopausal women with MBC and as second-line use after tamoxifen failure. Letrozole is more potent than anastrozole but results in no difference in overall survival.
• Direct comparison of tamoxifen to AIs as first-line therapy in three large randomized studies showed no statistically significant difference in median overall survival (8, 9). However, letrozole produced a longer time to progression (TTP) and a higher overall response rate (8). As a result, AIs are often preferred over tamoxifen as first-line therapy in postmenopausal women with MBC.
• Exemestane is a steroidal AI approved for second-line therapy in postmenopausal women with MBC. Exemestane may induce responses in patients with primary resistance to tamoxifen. Although not approved in the United States for first-line therapy in MBC, it is a reasonable choice; exemestane results in prolonged PFS and improved response rates compared to tamoxifen for first-line therapy (10) and has similar efficacy as nonsteroidal AIs.
• Everolimus is an oral rapamycin analogue that inhibits mTOR and is approved in combination with exemestane for ER-positive/HER2-negative MBC following progression on a prior nonsteroidal AI (11). Addition of everolimus resulted in improved PFS (11.0 vs. 4.1 months) and improved response rate (12.6% vs. 1.7%), although no statistically significant improvement in overall survival was observed and toxicity was significantly higher.
• Fulvestrant is approved for postmenopausal MBC and results in similar median survival and TTP as tamoxifen (12, 13). Fulvestrant is similar in efficacy and safety to exemestane following progression on a nonsteroidal AI (14). However, dose of fulvestrant used in prior studies was shown to be inferior to the currently recommended higher dose (15).
• Ovarian suppression (OvS) with the leutinizing hormone-releasing hormone (LHRH) agonists (goserelin, triptorelin, and leuprolide) has equal efficacy to surgical OvS for premenopausal women with MBC. LHRH agonists may cause a flare reaction similar to tamoxifen. Combined tamoxifen and OvS results in higher response rates than OvS alone (39% vs. 30%), without clear survival benefit over sequential therapy.
• Megestrol acetate may have a role in patients who progress on previous therapies but has largely been replaced by the antiestrogens and AIs.
• Estradiol administration at 6 mg orally may be active in ER-positive breast cancer that has previously been treated with antiestrogen therapy and may be an option for appropriate candidates (15, 16).
• In men with hormone receptor-positive MBC, tamoxifen is the preferred initial therapy. Aromatase inhibitors have limited data but do demonstrate activity in men who progress after tamoxifen. The role of GnRH agonists is unclear in men treated with endocrine therapy. Men who are resistant to endocrine therapy or are HER2+ should be treated according to the algorithms below.
 CHEMOTHERAPY
CHEMOTHERAPY
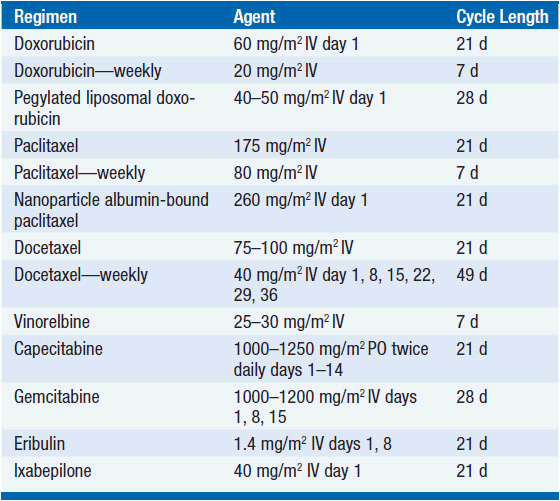
A treatment algorithm is shown in Figure 59-2 and representative common chemotherapy regimens for MBC are shown in Tables 59-2 through 59-4 (3, 9–14). The mechanism of action and toxicities of specific chemotherapies are described in more detail section 1, “Classes of Drugs.” The following are guidelines for chemotherapy treatment in MBC:
• Patients with visceral metastases, rapidly progressive disease, or hormone-refractory disease may be treated with chemotherapy.
• First-line chemotherapy results in response rates of 30%–60% and improved QOL.
• Combination chemotherapy has higher response rates but more toxicity and no survival benefit over sequential single agents. Second-line chemotherapy should use single agents except in selected patients.
• Factors predicting resistance to chemotherapy include relapse within 12 months of adjuvant chemotherapy, progression on a prior chemotherapy regimen, poor performance status, and multiple sites of visceral disease.
• Concurrent chemotherapy and hormonal therapy for MBC should be avoided.
• Several first-line therapy options are considered reasonable, and the choice of agent should be individualized to balance efficacy, toxicity, and breast cancer subtypes. Such agents include anthracyclines, taxanes, capecitabine, and platinums.
• Liposomal doxorubicin has equal efficacy to standard doxorubicin (17) but allows for increased cumulative dosing.
• Paclitaxel and docetaxel may be given on weekly or every 3-week schedules. Response rates may be higher with weekly dosing, but overall survival is similar and neurotoxicity is significantly worse. Patients who progress on paclitaxel may respond to docetaxel.
• Nanoparticle albumin-bound (nab)-paclitaxel is approved for treatment of MBC. Benefits include treatment without steroid premedication, shorter infusion times, higher effective administered paclitaxel dose, and lower hematologic toxicity. No difference in overall survival has been shown (18).
• Capecitabine, an oral 5-FU derivative, has response rates up to 28% as monotherapy. The combination of capecitabine and docetaxel showed improved survival (14.5 vs. 11.5 months) compared to single agent docetaxel in patients who progress after anthracycline therapy (19). Intravenous 5-fluorouracil is used in many of the common combination regimens for MBC but rarely as a single agent.
• Single agent vinorelbine has response rates up to 25%–50% and is particularly well tolerated in elderly patients.
• Eribulin is a novel non-taxane antimicrotubule agent recently approved for MBC. In a phase III trial comparing eribulin to physician’s treatment choice in MBC patients who received between 2 and 5 prior lines of therapy, overall survival was extended by nearly 3 months (HR 0.81) and a response rate increased from 5% to 12% (20). The short infusion time, lack of required premedication, and generally tolerable side-effect profile have been attractive features for its use in MBC.
• Single agent gemcitabine is well tolerated and has response rates up to 40% in chemotherapy-naive patients. Combination with cisplatin, vinorelbine, or paclitaxel results in higher response rates, but also increased toxicity with no improvement in overall survival. Gemcitabine may have a role in third-line therapy and beyond.
• Ixabepilone is a novel epothilone microtubule inhibitor approved as a single agent for MBC after prior anthracycline, taxane, and capecitabine. It is also approved in combination with capecitabine after anthracycline and taxane treatment (21, 22). Response rates are higher in combination with capecitabine (35%–43%) than as monotherapy (12%). However, despite an improved PFS, no improvement in overall survival is seen with the combination over capecitabine monotherapy.
• Bevacizumab is a monoclonal antibody against vascular endothelial growth factor (VEGF). First-line therapy using paclitaxel plus bevacizumab compared to paclitaxel monotherapy showed a significant improvement in PFS (11.4 vs. 6.1 months) (23). No difference in overall survival was observed. However, subsequent studies reported less robust findings and in 2011 the FDA revoked the breast cancer indication for bevacizumab. The role of bevacizumab in MBC remains undefined and its use with chemotherapy remains controversial. Significant efforts are ongoing to identify biomarkers of response and to identify clinical situations where bevacizumab may be most active.
• Supportive measures should be considered in patients with an ECOG performance status ≥3 or after lack of response to three successive chemotherapy regimens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree