Metabolic Emergencies in Oncology
INTRODUCTION
Metabolic emergencies represent rare events in the care of cancer patients. In contrast to the measured pace of most of oncology, these scenarios demand prompt identification and intervention. This chapter reviews tumor lysis syndrome, hyponatremia, and hypercalcemia.
TUMOR LYSIS SYNDROME
 DEFINITION
DEFINITION
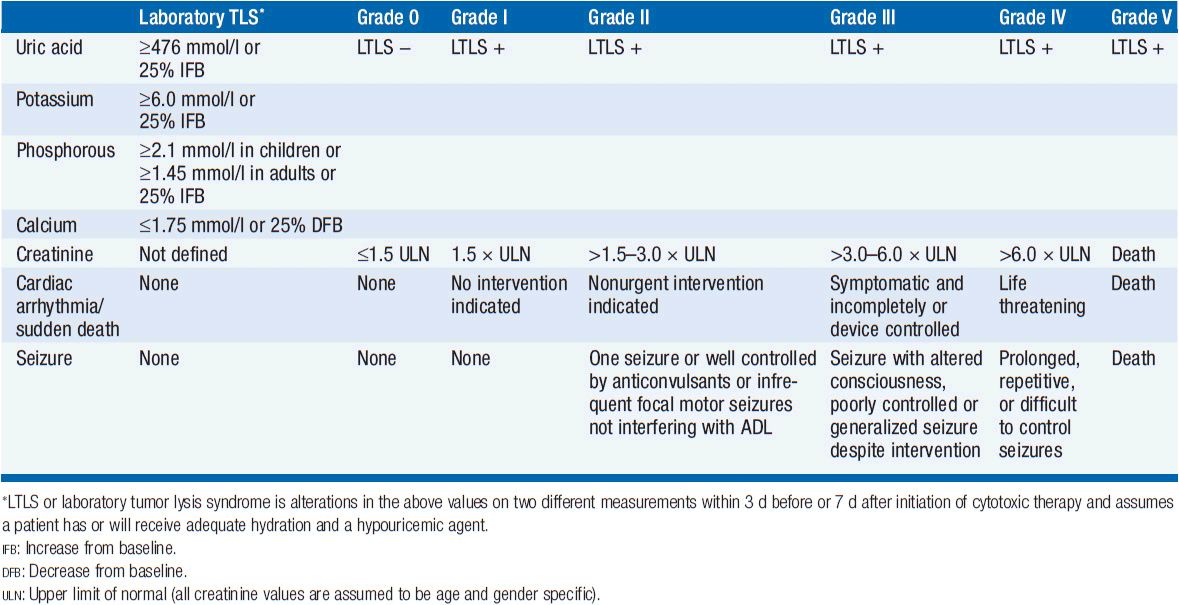
Tumor lysis syndrome is a collection of metabolic derangements secondary to the release of tumor cell contents into the extracellular space (Table 20-1). The rapid development of hyperphosphatemia, hyperuricemia, and hyperkalemia can cause devastating renal and cardiac complications. Hypocalcemia occurs secondarily as a consequence of hyperphosphatemia.
Tumor lysis syndrome occurs most frequently in rapidly growing hematologic malignancies such as acute leukemias and Burkitt’s lymphoma. In these diseases, it can occur either spontaneously or more commonly 6–72 h following initiation of antitumor therapy. There are case reports of even more rapid onset with the use of targeted therapies. The syndrome is most frequently associated with cytoxic chemotherapy but can occur after embolization, radiation, or glucocorticoids. The Cairo-Bishop definitions and grading system for laboratory and clinical tumor lysis syndrome are not widely used in clinical practice (Table 20-2) (1). The National Cancer Institute Common Toxicity Criteria 4.0 system grade TLS by its presence (grade 3), life threatening consequences (grade 4), or death (grade 5).
 INCIDENCE AND RISK FACTORS
INCIDENCE AND RISK FACTORS
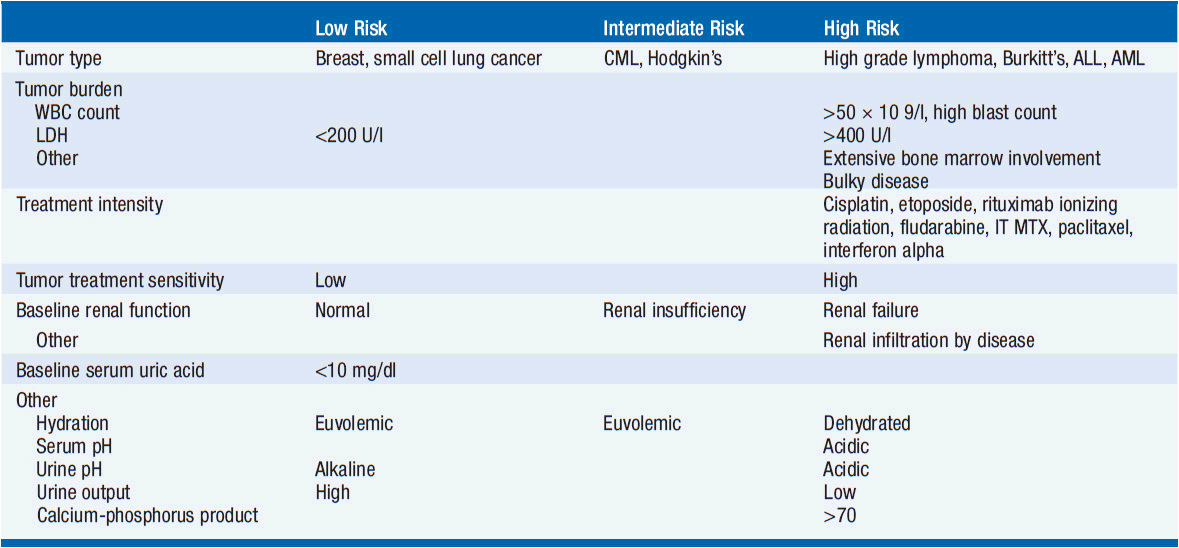
There are both tumor-related risk factors for tumor lysis syndrome, as well as patient-specific risks. Tumor-specific risks include a large burden of disease, a high-proliferative rate, or a highly treatment-sensitive tumor. Clinically significant tumor lysis syndrome is estimated to occur in up to 30% of patients with Burkitt’s lymphoma/B-ALL and 15% of patients with acute myeloid leukemia, although laboratory evidence of tumor lysis syndrome can be present in a larger proportion of patients. There is a significant but lower risk in diffuse large B-cell lymphoma, and rarer reports in chronic lymphocytic leukemia, and multiple myeloma. The use of the monoclonal antibody rituximab has been associated with the development of tumor lysis syndrome in patients with increased numbers of circulating tumor cells (≥25,000) or a large tumor burden. Tumor lysis syndrome is rare in solid tumors, with breast cancer and small cell lung cancer comprising the most common reports. There are case reports of tumor lysis syndrome with a number of targeted therapies (Table 20-3).
Patient comorbidities increase the risk of tumor lysis syndrome. Decreased urinary flow or dehydration, chronic renal insufficiency or frank renal failure, acidic urine, or preexisting hyperuricemia are all risk factors for tumor lysis syndrome development.
 MECHANISMS
MECHANISMS
Purine Catabolism
The major contributing element in the pathophysiology of tumor lysis syndrome is hyperuricemia caused by the release of nucleic acids (purines), which are catabolized to uric acid, leading to elevated plasma urate levels (Figure 20-1). Elevated uric acid levels overwhelm the excretory capacity of the renal tubules particularly in the presence of an acidic pH and low urine flow. Crystalline obstruction of the renal tubules, uric acid nephropathy, renal failure, and uremia may result. Renal failure may also occur due to volume depletion and changes in autoregulation within the kidney.
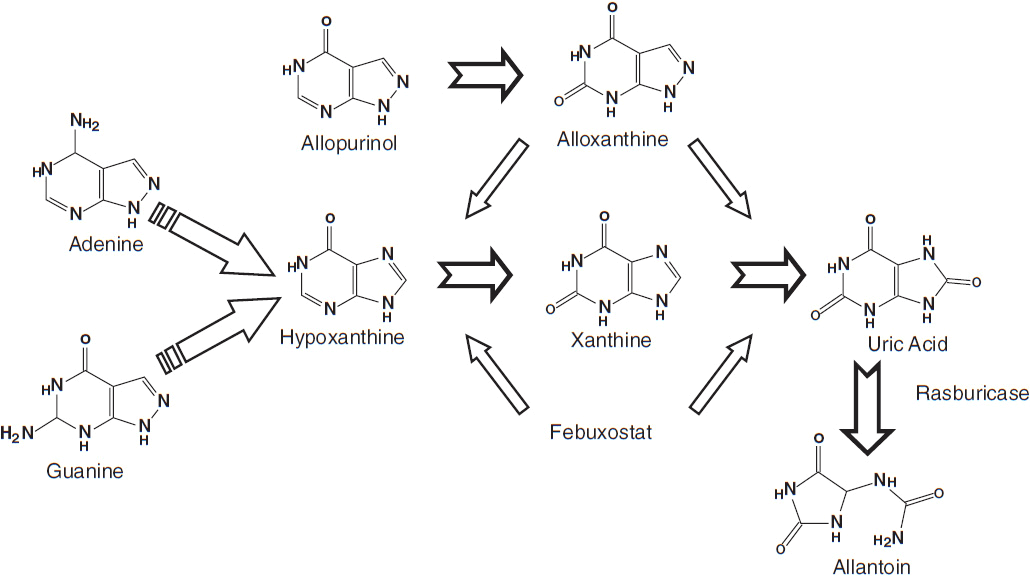
FIGURE 20-1 Uric acid formation via purine catabolism and the mechanism of action of allopurinol, rasburicase, and febuxostat.
Intracellular Ion Release
Other intracellular ions are released, including potassium and phosphorus. Hyperkalemia-induced cardiac arrhythmias exacerbated by renal failure are the most life-threatening complication of tumor lysis syndrome.
Intracellular phosphorous, which may be overproduced in malignant cells, is also released, renally excreted, and may precipitate as calcium phosphate in the renal tubules, leading to hypocalcemia, metastatic calcification, intrarenal calcification, nephrocalcinosis, nephrolithiasis, and obstructive uropathy.
Hypocalcemia is generally due to precipitation of calcium phosphate in the soft tissues and kidneys during periods of hyperphosphatemia and inappropriately low levels of 1, 25-dihydroxyvitamin D3. This can be clinically associated with tetany and cardiac arrhythmias.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
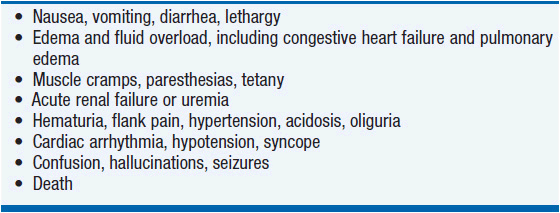
The clinical presentation of tumor lysis syndrome is characterized by the symptoms of the individual electrolyte imbalances and resultant renal failure (Table 20-4).
 DIFFERENTIAL DIAGNOSIS
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of tumor lysis syndrome is limited. Injuries to large structures such as ischemic limb or rhabdomyolysis can replicate the varied clinical and laboratory presentations of tumor lysis syndrome but should be clinically apparent. The other laboratory abnormalities may be present to some extent in patients with renal insufficiency from any cause (Table 20-5).
 THERAPEUTIC AGENTS, MONITORING
THERAPEUTIC AGENTS, MONITORING
See Tables 20-3 and 20-6 for an overview of the following discussion. Patient- and tumor-specific factors are combined in a stratification schema that divides patients into high, intermediate, and low-risk cohorts. High-risk patients include all patients with Burkitt’s lymphoma, lymphoblastic lymphoma, or B-cell ALL as well as patients with ALL with a WBC ≥100,000, AML with WBC ≥50,000, or monoblastic subtype. Intermediate-risk patients include all diffuse large B-cell lymphoma, ALL with WBC 50–100,000, AML with WBC 10–50,000, and CLL with WBC ≥10,000 treated with fludarabine as well as all other rapidly proliferating malignancies with an expected rapid response to therapy. Low-risk patients include all indolent lymphomas as well as ALL with a WBC ≤50,000, AML and CLL with WBC ≤10,000 (2).
In patients at high risk of developing tumor lysis syndrome, the decision to delay therapy must be weighed against the risks of tumor lysis syndrome, but in any case prophylactic measures should be instituted. Drugs that contribute to electrolyte abnormalities are nephrotoxic, or block uric acid, or potassium excretion should be stopped.
Hydration and Diuresis
The mainstay of prevention is aggressive hydration and diuresis, with individuals receiving 2–4 times usual maintenance fluids or 3–6 l/m2/day. These fluids should not initially include potassium, calcium, or phosphate. If the patient is not hypovolemic but is oligo or anuric, diuretics such as furosemide (or mannitol, particularly if furosemide fails) may be used to maintain an appropriate urine output with the following as goals: urine specific gravity should be <1.010 and urine output >150–250 ml/h. Furosemide diuresis may also decrease serum potassium levels. Dosing of diuretics is patient dependent and may be intermittent or continuous.
Traditionally, recommendations have also suggested alkalinization of urine with sodium bicarbonate. However, this is not recommended in the era of recombinant urate oxidase due to the increased risk of urinary xanthine and calcium phosphate deposition, fluid overload, and metabolic alkalosis (3).
Allopurinol
Allopurinol, a xanthine analog that is converted to alloxanthine and acts as a competitive inhibitor of xanthine oxidase, reduces the production of uric acid and the incidence of related obstructive uropathy (Figure 20-1). It does not reduce levels of uric acid already present and leads to lower urate levels over days. Allopurinol may also result in xanthine nephropathy and calculi due to accumulation of xanthine and hypoxanthine, which are renally excreted.
Usual dosing of allopurinol is 2 mg/m2/day or 600–800 mg/day orally, 12 h to 3 days prior to initiation of chemotherapy (or 200–400 mg/m2/day IV, 24–48 h before initiation of chemotherapy; maximum dose 600 mg/day if unable to take orally). Allopurinol should be renally dosed in appropriate patients. The intravenous and oral forms have equivalent efficacy.
Allopurinol also reduces the degradation of other purines including 6-mercaptopurine (6-MP) and azothioprine, and therefore, these drugs should be dose-reduced when used with allopurinol.
Rasburicase
Rasburicase (Elitek) is a recombinant urate oxidase that converts uric acid to allantoin, a more soluble compound, which is then renally excreted (Figure 20-1). Usual dosing is 0.20 mg/kg IV over 30 min for 1–5 days and does not require renal dosing. Side effects include anaphylaxis or hypersensitivity reactions (bronchospasm, hypoxemia) in 5% of those who receive this drug. In addition, patients may form antibodies to this drug, the significance of which is currently unknown. Rasburicase should not be used in patients with G-6PD deficiency as hemolytic anemia and methemoglobinemia may occur. In the majority of patients, a single dose is sufficient to control hyperuricemia.
A randomized trial comparing rasburicase, allopurinol, and the combination of the two agents in adult and pediatric patients at high risk for tumor lysis syndrome demonstrated more rapid control of uric acid in a higher percentage of patients in the group treated with rasburicase alone.
Febuxostat
Febuxostat is a nonpurine selective inhibitor of xanthine oxidase used for prevention of gout. A phase 3 trial for tumor lysis syndrome prevention is currently ongoing.
Monitoring
Monitoring for tumor lysis involves twice daily serum electrolytes (more frequently if the patient is at high risk or clinically unstable), and at least daily urinary pH and spot uric acid to creatinine ratio (value should be >1.0), evaluation of volume status (including daily weights, blood pressure, and examination looking for edema, pleural effusions, and ascites), and serum WBC count, LDH, ionized or corrected calcium, and calcium phosphorus product.
ECG changes associated with hyperkalemia include widening of the QRS complex (late manifestations include a sine wave appearance, ventricular arrhythmias, or asystole) and peaked T waves, while hypocalcemia is associated with a long QT secondary to a prolonged ST segment.
 TREATMENT
TREATMENT
Hyperkalemia
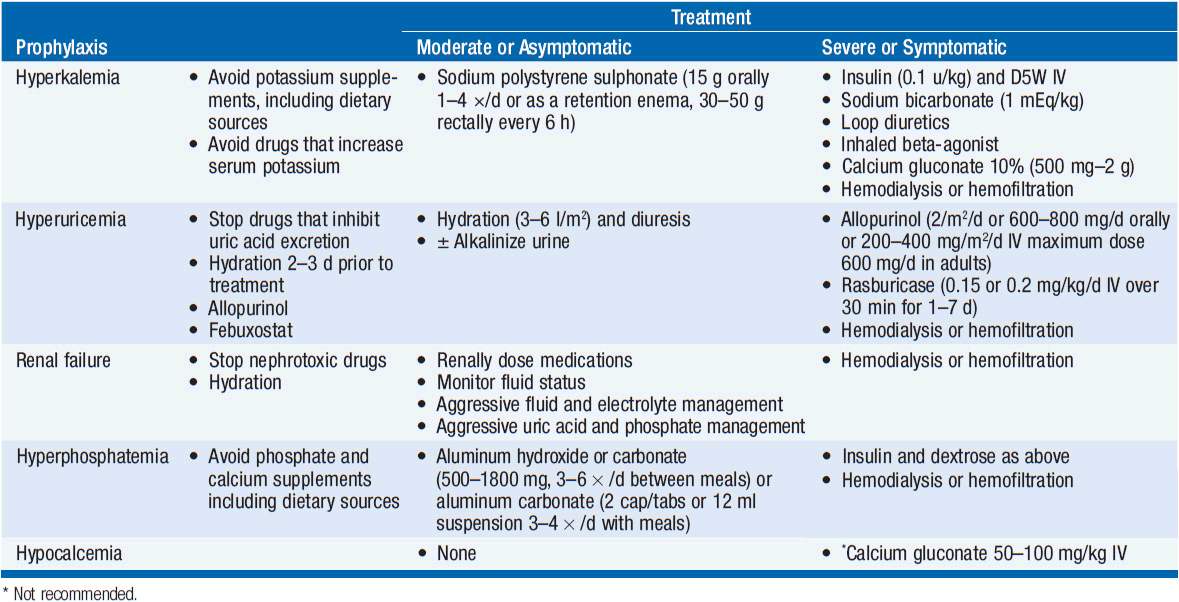
Hyperkalemia treatment can be divided into treatment for moderate or asymptomatic hyperkalemia and those for severe or symptomatic hyperkalemia (Table 20-6).
For asymptomatic or moderate hyperkalemia, oral sodium polystyrene sulphonate (Kayexalate), an exchange resin, is the primary treatment (15 g orally 1–4 times daily as a slurry in water or 70% sorbitol or as a retention enema, 30–50 g rectally every 6 h as a warm emulsion in 100 ml aqueous vehicle (sorbitol or D20W), retained for 30–60 min in adults).
For severe cases, Kayexalate in addition to insulin and dextrose, sodium bicarbonate (1 mEq/kg, repeated as needed based on blood gas values), loop diuretics, inhaled beta-agonists, and, in extreme examples, calcium gluconate or chloride is appropriate. For calcium gluconate, the dosage is 2–4 mg/kg of a 10% solution, repeated at 10-min intervals as necessary. Calcium chloride is reserved for emergency situations. Hemodialyis or hemofiltration should also be considered.
Hyperuricemia And Renal Failure
Hyperuricemia should be treated with rasburicase. Hemodialysis or hemofiltration may be necessary.
Renal failure requires aggressive fluid, electrolyte, and uric acid management along with close monitoring for indications requiring hemodialysis or hemofiltration. Hemodialysis indications may include fluid overload, metabolic acidosis, electrolyte disturbances that are recalcitrant to the treatments outlined above, uremia, and hypertension.
Hyperphosphatemia
Hyperphosphatemia is treated with oral aluminum hydroxide (adult dosing: 500–1800 mg, 3–6 times daily between meals) or aluminum carbonate (2 capsules or tablets or 12 ml suspension 3–4 times daily with meals). Insulin and dextrose as administered in hyperkalemia can also be given. Severe or symptomatic cases should receive hemodialysis or hemofiltration.
Hypocalcemia
Moderate or asymptomatic hypocalcemia usually does not require treatment, and the risks of treatment may outweigh the benefits. In severe cases, calcium gluconate 500 mg to 2 g (10% solution) IV in adults at a rate not to exceed 1.5 ml/min can be used but may lead to calcium phosphate precipitation resulting obstructive uropathy or metastatic calcification. In an emergency situation, calcium chloride should be used for hyperkalemia or hypocalcemia at 2–4 mg/kg of a 10% solution, repeated at 10-min intervals as necessary.
HYPONATREMIA
 INCIDENCE AND RISK FACTORS
INCIDENCE AND RISK FACTORS
Hyponatremia is a common electrolyte abnormality, occurring in many elderly and/or hospitalized patients. In cancer patients, hyponatremia may be due to primary or metastatic disease, cancer-related medical interventions or complications, usually via the syndrome of inappropriate antidiuretic hormone (SIADH), as well as other causes. This section will focus primarily on the diagnosis and treatment of SIADH-related hyponatremia in the setting of malignancy (4).
Malignancy-related hyponatremia is most commonly seen in lung carcinomas, particularly the small cell variant. However, head and neck cancers, leukemia, and mediastinal cancers have also been implicated, along with limited reports in a number of other cancers. Hyponatremia can also be therapy related and has been reported after stem cell transplantation.
 MECHANISMS
MECHANISMS
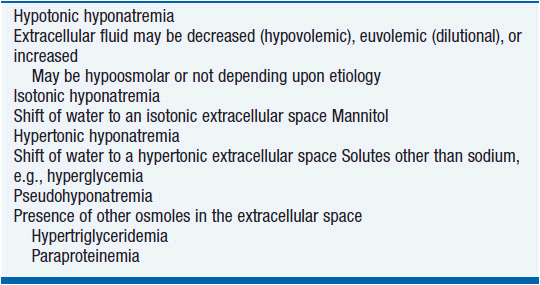
Hyponatremia can be characterized by varying levels of osmolality (Table 20-7). Hypotonic hyponatremia, the most common form, is caused by abnormal retention of water with varying amounts of fluid intake (Table 20-8). Retention of water occurs because the ability of the kidneys to excrete fluid has been impaired or overwhelmed. Edema of the central nervous system is the most concerning consequence of hyponatremia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree