Metabolic Bone Diseases
Susan V. Bukata
Metabolic bone diseases include a variety of diseases that affect the strength and overall quality of bones. Some of these diseases affect a huge proportion of the population. Osteoporosis alone affects 45% of women over age 50. Other diseases are extremely rare and usually result from a genetic anomaly that affects normal bone metabolism. In every metabolic bone disease there is an imbalance in the cells and pathways that allow for the skeleton to be continually remodeled throughout one’s lifetime.
Pathophysiology Fundamentals
Bone metabolism is an integral part of the endocrine system.
Three cell types are involved:
Osteoblasts
Originate from mesenchymal cells
Synthesize organic bone matrix
Bear receptors for parathyroid hormone and hormones, including estrogen
Produce osteoprotegerin (OPG) and receptor activator of nuclear factor (NF)-kappa B (RANK) ligand
Produce alkaline phosphatase (marker for bone formation)
Osteoclasts
Originate from monocyte precursors
Recruitment/development/activity signals through RANK ligand and macrophage colony-stimulating factor (M-CSF)
Resorb bone at ruffled membrane
Secrete protons/lysosomal enzymes
Osteocytes
Derived from osteoblasts encased in matrix
Interconnected through cytoplasmic processes
No longer form bone
Respond to mechanical signals and influence remodeling
RANK/RANK-Ligand Signaling
Responsible for the coordination between osteoblasts and osteoclasts; plays an important role in bone metabolism. The osteoblast is the cell controlling this pathway.
RANK-ligand (RANK-L) signal on surface of osteoblasts and secreted by them
RANK receptor on osteoclast
OPG is inhibitor of RANK-L (blocks binding to RANK).
Bone Metabolic Unit
Osteoclastic bone resorption and osteoblastic bone formation
Concept of coupling
Resorption > formation leads to bone loss.
Resorption < formation leads to bone gain.
Resorption = formation leaves bone mass balanced.
Peak bone mass reached at age 25 to 30. After that, resorption is slightly greater than formation, leading to slow bone mass loss.
Calcium
Regulation is extremely important in bone mass maintenance.
Intestines/kidneys/bone involved in calcium metabolism
Bone first source for calcium when needed (99% of body store is there)
Active absorption in duodenum (calcium binding protein)
Passive absorption in jejunum
Calcium balanced when renal excretion = intestinal absorption
Renal reabsorption
Actively in distal convoluted tubule
Passively in proximal tubule and loop of Henle
Dietary intake requirement varies with age.
Adolescents age 9 to 18 need >1,300 mg daily.
Age 19 to 49 need >1,000 mg daily.
Older adults >50 need >1,200 mg daily.
Drugs that decrease calcium retention (thus increase calcium loss)
Furosemide (Lasix)
Heparin
Corticosteroids
Tetracycline
Drugs that increase calcium retention
Hydrochlorothiazide (HCTZ): can be used to help retain calcium through renal channels in patients with high urinary loss
Vitamin D
Important for calcium regulation and bone health
Fat-soluble steroid hormone
Sources
Diet (vitamin D2)
Endogenous production in skin (vitamin D3)
Hydroxylated in liver (at 25th carbon), then kidney (at 1st carbon) to create 1,25-dihydroxyvitamin D
25-hydroxyvitamin D also recognized as important in maintaining bone health
Levels >30 ng/dL desired
Targets
Kidney: increases resorption in proximal tubule
Intestines: regulates production of calcium binding protein
Bone: major target enhancing mobilization of calcium stores
Receptors on osteoblasts stimulate RANK ligand production and therefore osteoclast development and activity.
Recommended daily intake: 400 to 800 IU daily for adults
Parathyroid Hormone
Controls regulation of serum calcium levels
Calcium-sensing receptor on parathyroid cells initiates hormone release with low serum calcium levels.
Bone: PTH binds to osteoblast receptors
Neutral protease release initiates bone remodeling.
Stimulates production of factors that signal osteoclasts to resorb bone.
Kidney
Proximal tubule: PTH decreases PO4 resorption
Distal tubule: PTH increases calcium resorption
Stimulates 1α hydroxylase to increase 1,25-vitamin D levels
Intestine
Increases calcium binding protein production to increase calcium absorption
Greatest quantitative effect on calcium
PTHrP production by some cancers with similar effects
Osteoporosis
Osteoporosis is a metabolic bone disease characterized by low bone mass and a microarchitectural deterioration of bone tissue that results in enhanced bone fragility and a consequent increase in fracture risk.
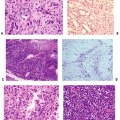
Pathophysiology
Imbalance in bone metabolic unit between osteoclastic bone resorption and osteoblastic bone formation
Resorption > formation leads to bone loss.
Peak bone mass reached at age 25 to 30
After age 30, resorption is slightly greater than formation.
Can see rapid increase in bone resorption during menopause
Can see 30% bone mass loss over perimenopausal period
Epidemiology
Etiology is unknown.
Affects 45% of women and 25% of men aged 50 and older
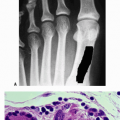
Osteoporotic fractures
4 times more common than stroke
Having one is a major risk factor for subsequent fractures.
10% have another fragility fracture in <1 year.
17% to 21% have another fragility fracture in <2 years.
Pose a lifetime risk of death comparable to breast cancer
1 in 3 women and 1 in 6 men will suffer a hip fracture.
Annual hip fractures
United States: >300,000
Europe: >400,000
Incidence expected to double over the next 50 years.
Surgeon General’s report in 2004 recognizes poor bone health as an epidemic and major health crisis.
Personal cost of fracture
Quality of life
Economic costs of fracture
Risk factors for osteoporosis
Genetic
Female > male
Caucasian or Asian > Hispanic or African American
Environmental
Smoking
Alcohol
Sedentary lifestyle
Low body weight (<85% ideal body weight or <120 lbs)
History of eating disorder
Other
Personal/family history of fragility fracture
Age >50
Classification
High-turnover osteoporosis
Primary form at menopause, but can be seen at any age and in men
Enhanced osteoclastic bone resorption with more and deeper lacunae
Osteoblasts unable to fully replace resorbed bone
Elevated bone turnover markers
Bone loss rate can be 2% to 3% per year, lasting 6 to 10 years.
Low-turnover osteoporosis
Most commonly seen with aging, but can be seen at any age
Failure of osteoblasts to form bone
Bone formation markers show decreased levels (not good bone formers); osteoclastic bone resorption is normal or slightly decreased.
Bone turnover markers at premenopausal level or lower
Can also be seen in individuals with underlying genetic collagen disorder
Diagnosis
Clinical Features
Biochemical markers
Collagen cross-link products measured for bone loss rate
Urine N-telopeptide
Measure in any urine except the first of the day
Generally want to have value <30 nM BCE/mM creatinine, definitely <40 nM BCE/mM in postmenopausal women and older men
Expect marker level to go down with bisphosphonate treatment by at least 30% to 40% from baseline.
Serum cross laps
Diurnal variation for each individual
More commonly used in Europe
Markers for bone formation (low levels = poor bone formation)
Osteocalcin
Alkaline phosphatase
Get bone-specific alkaline phosphatase (BSAP), or also need liver function enzymes to evaluate if liver activity is elevated.
All biochemical markers are elevated in the setting of a healing fracture and then return to baseline.
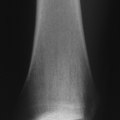
Radiologic Features
Dual-Energy X-Ray Absorptiometry {lDXA}
Currently gold standard
Low radiation doses (1 to 3 mrem), short scanning times
Error range from 3% to 5% between serial scans on same machine, can be greater between scans on different machines
DXA scan scoring (matching race and gender)
T-score
Compares density relative to peak bone mass (normal healthy 25-year-old)
Score used to determine level of disease over age 25
Z-score
Compares density to peers of same age
Measurement used for children and adults up to age 25
Quantitative Computed Tomography (CT)
More radiation exposure, more operator-dependent
Assesses both trabecular and cortical areas separately
Use hydroxyapatite phantom for calculating density.
Ultrasound
May be a good tool for preliminary screening
Can evaluate only subcutaneous bones (calcaneus/tibia)
Fracture risk at hip/spine not highly correlated (only 70%)
World Health Organization Definitions of Osteoporosis and Osteopenic
Bone mass measured at hip and spine for adults
Defined from lower of two levels
Total body and spine measured for children
1 to 2.4 standard deviations below peak bone mass (T = -1.0 to -2.4)
Osteopenic with range of mild to moderate bone deficiency
>2.5 standard deviations below peak mass (T = -2.5 or lower)
Osteoporotic
Fragility fracture defines as osteoporotic regardless of T-score
Treatment
Prevention
Attainment of peak bone mass (age 20 to 30)
Prevention of postmenopausal resorption and agerelated bone loss (Table 10-1)
Table 10-1 Prevention of Postmenopausal Resorption and Age-Related Bone Loss
Treatment
Dose
Side Effects
Issues with Treatment
Mechanism of Action
Oral Bisphosphonates
Fosamax (alendronate)
Actonel (risedronate)
Boniva (ibandronate)
70 mg/wk
35 mg/wk
150 mg/mo
Reflux
GI distress
Myalgias and bone pain in early doses
GI bleeding and esophageal erosions
Poor absorption
Renal clearance of intact drug (need good renal function or drug accumulates)
Affects osteoclast function and number
Stops bone loss
IV Bisphosphonates
Aredia (pamidronate)
Zometa (zoledronic acid)
Boniva (ibandronate)
90 mg q 3 mo
4 mg/yr
3 mg q 3 mo
Myalgias and bone pain with initial doses
Rare cases of osteonecrosis of the jaw
Affects osteoclast function and number
Stops bone loss
SERM (selective estrogen receptor modulator)
Evista (Raloxifene)
60 mg/day
Leg cramps
Hot flashes
Increased risk of deep vein thrombosis in first 4 months of dosing
Cardiovascular neutral
Breast cancer protective
Use only in postmenopausal women
Returns bone dynamics to premenopausal pattern
Stops bone loss
Estrogen (with progesterone)
Prempro
0.625 mg/2.5 mg
0.45 mg/1.5 mg
0.3 mg/1.5 mg
Persistent menstrual bleeding
Increased risk heart attack, stroke, pulmonary embolus, invasive breast cancer
Use lowest effective dose to manage postmenopausal symptoms.
Return to premenopausal bone dynamics
Protection against hip and vertebral fracture
Estrogen
Premarin
0.625 mg
0.45 mg
0.3 mg
1.25 mg
0.9 mg
Increased risk of stroke
Increased risk of endometrial cancer in women with intact uterus
No increased risk of breast cancer
Bone benefits equal at all doses; use lowest effective dose for other symptoms
Return to premenopausal bone dynamics
Protection against hip and vertebral fracture
1-34 PTH
Forteo (teriparatide)
20 mcg/day SC for maximum of 2 yr
Dizziness and myalgias in first 4 to 6 weeks of use in some patients
Black Box Warning with increased rate of osteosarcoma in rats
Stimulates osteoblastic bone formation greater than osteoclastic bone resorption 
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree