Abstract
With the increase in life expectancy, a woman may now expect to spend one-third or more of her life after menopause. Thus the understanding of the physiology of menopause and aging and possible management strategies assumes great significance for women’s health. This review will first discuss the epidemiology of menopause, early or premature menopause, and the effects of menopause on various organ systems and diseases that occur after menopause. This will be followed by a consideration of various therapies for the symptoms of menopause, osteoporosis, and various preventative strategies for women after menopause. Hormonal and nonhormonal therapies will be discussed.
Key words
Menopause, premature ovarian insufficiency, estrogens, androgens, gonadotropins, AMH, inhibin, vulvovaginal atrophy, osteoporosis, coronary disease, stroke, venous thromboembolism, mortality, prevention, hormone therapy, nonhormonal treatment
Epidemiology
- ◆
The age of menopause has been constant over several centuries and is influenced by genetic, ethnic, and environmental variables.
- ◆
The prediction of the age of menopause based on anti-müllerian hormone (AMH) trajectories is possible but impractical.
Menopause is defined by the last menstrual period. Because cessation of menses is variable and many of the symptoms thought to be related to menopause may occur prior to the cessation of menses, there is seldom a precise timing of this event. Other terms used are perimenopause , which refers to a variable time beginning a few years before and continuing after the event of menopause, and climacteric , which merely refers to the time after the cessation of reproductive function. While the terms menopausal and postmenopausal are used interchangeably, the former term is less correct because “menopausal” should only relate to the time around the cessation of menses.
As life expectancy increases beyond the eighth decade worldwide, particularly in developed countries, an increasing proportion of the female population is postmenopausal. With the average age of menopause being at 51 years old, more than one-third of a woman’s life is now spent after menopause. Here symptoms and signs of estrogen deficiency merge with issues encountered with natural aging. As the world population increases and a larger proportion of this population is made up of individuals over 50, medical care specifically directed at postmenopausal women becomes an important aspect of modern medicine. Between the years 2000 and 2005, the world population older than 60 years old increased from 590 million to 1 billion. In the United States, the number of women entering menopause will almost double in the 30 years between 1990 and 2020 ( Table 14.1 ).
| Year | Population |
|---|---|
| 1990 | 10.8 million |
| 2000 | 12.1 million |
| 2010 | 17.1 million |
| 2020 | 19.3 million |
Age of menopause, which is a genetically programmed event, is subject to some variability. The age of menopause in Western countries (between 51 and 52 years old) is thought to correlate with general health status. Socioeconomic status is associated with an earlier age of menopause. Higher parity, on the other hand, has been found to be associated with a later menopause. Smoking has consistently been found to be associated with menopause onset taking place 1 to 2 years earlier. While body mass has been thought to be related to age of menopause (greater mass with later menopause), the data have not been consistent. Malnourishment and vegetarianism have both been found to be associated with earlier onset of menopause. However, physical and athletic activity has not been found consistently to influence the age of menopause.
There also appear to be ethnic differences in the onset of menopause. In the United States, African-American and Hispanic women have been found to have menopause approximately 2 years earlier than white women. Although parity is generally greater around the world than in the United States, the age of menopause appears to be somewhat earlier. Malay women have menopause at approximately age 45, Thai women at age 49.5, and Filipina women between the ages of 47 and 48. Indian women have also been reported to have menopause by age 46.2 years. Women in countries at higher altitude (Himalayas or Andes) have been shown to have menopause 1 to 1.5 years earlier. Because the average age of menopause in the United States is 51 to 53 years with an age distribution weighted toward white women, menopause prior to age 40 is considered premature. Conversely, by age 58, 97% of women will have gone through menopause.
The primary determinate of age of menopause is genetic. Based on family studies, heritability for the age of menopause averaged 0.87—suggesting that genetics explain up to 87% of the variance in menopausal age. Prediction models for the age of menopause have been based on the downward trajectory of values of AMH with age, as shown in several studies, noted below. In a recent review of the literature, maternal age of menopause does contribute somewhat to values of AMH in the prediction model, but AMH is thought to the most valuable marker.
Multiple genic loci have been identified by genome-wide association studies, which are associated with the age of natural menopause as well as ovarian insufficiency. In a meta-analysis of 22 genome-wide association studies, candidate genes identified appear to be involved in DNA replication and damage repair, hormone production and action, and immune function. These include EXO1, HELQ, U1MC1, FAM175A, FANCI, TLK1, POLG, MCM8, and PRIM1 associated with DNA repair , and IL11, NLRP11, and PRRC2A in the immune function domain. Nevertheless, in spite of these advances, the mechanisms of action of these genes and their interactions with environmental factors and possible gonadotoxic factors, which may affect ovarian reserve and reproductive lifespan, remain to be determined.
Premature Ovarian Failure
- ◆
Premature ovarian failure (POF) or insufficiency occurs in up to 10% of young amenorrheic women and has multiple etiologies, but is often idiopathic.
- ◆
Treatment is dependent on presenting symptoms.
- ◆
Spontaneous pregnancies may occur and long-term management should include estrogen replacement.
POF is defined as hypergonadotropic ovarian failure occurring prior to age 40. It has been suggested that women with POF should be referred to as having premature ovarian insufficiency (POI) to more effectively reflect its heterogeneous nature. In practice, both these terms are used interchangeably. POF has occurred in 5% to 10% of women who are evaluated for amenorrhea ; thus, the incidence varies according to the prevalence of amenorrhea in various populations. Estimates of the overall prevalence of POF in the general population range between 0.3% and 0.9% of women.
Throughout life, there is an ongoing rate of atresia of oocytes (see Chapter 8 ). Because this process is accelerated with various forms of gonadal dysgenesis due to defective X chromosomes, one possible etiology of POF is an increased rate of atresia that has yet to be explained. A decreased germ cell endowment or an increased rate of germ cell destruction can also explain POF. Nevertheless, some 1000 (of the original 2 million) primary follicles may remain. While most of these oocytes are likely to be functionally deficient, occasionally spontaneous pregnancies occur in young women in the first few years after the diagnosis of POF.
There are several possible etiologies of POF ( Box 14.1 ). Defects in the X chromosome may result in various types of gonadal dysgenesis with varied times of expression of ovarian failure. Even patients with classical gonadal dysgenesis (e.g., 45,XO) may undergo a normal puberty, and occasionally a pregnancy may ensue as a result of genetic mosaicism. Very small defects in the X chromosome may be sufficient to cause POF. Familial forms of POF may be related to either autosomal-dominant or sex-linked modes of inheritance.
Genetic
Enzymatic
Immune
Gonadotropin defects
Ovarian insults
Idiopathic
Mutations in the gene encoding the follicle-stimulating hormone (FSH)-receptor (e.g., mutation in exon 7 in the gene on chromosome 2p) have been described, but these are extremely rare outside of the Finnish population in which these mutations were originally described.
An expansion of a trinucleotide repeat sequence in the first exon on the FMR1 gene (Xq 27.3) leads to fragile X syndrome, a major cause of developmental disabilities in males. The permutation in fragile X syndrome has been shown to be associated with POF. There is a spectrum in findings based on the number of repeats in the premutation, and women with an “intermediate” number of repeats may have a lower ovarian reserve, which is often identified during an investigation for infertility.
Type 1 blepharophimosis/ptosis/epicanthus inversus syndrome, an autosomal-dominant disorder due to mutations in the forkhead transcription factor FOXL2, includes POF. Triple X syndrome has been associated with POF, and so has dystrophic myotonia, although the mechanism underlying this relationship is unclear.
Under the category of enzymatic defects, galactosemia is a major cause of POF that is related to the toxic buildup of galactose in women who are unable to metabolize the sugar. Even in women with fairly well-controlled galactose-free diets, POF tends to occur. Another enzymatic defect linked to POF is 17α-hydroxylase deficiency. This rare condition manifests differently from the other causes discussed here because the defect in the production of sex steroids leads to sexual infantilism and hypertension.
Because of the prevalence of autoimmune disorders in women, the degree to which autoimmunity may be responsible for POF is unclear. One study has suggested an association in 17.5% of cases. Virtually all autoimmune disorders have been found to be associated with POF, including autoimmune polyendocrinopathies like autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, which is caused by mutations in the autoimmune (AIRE) gene on 21q22.3. The presence of the thymus gland appears to be required for normal ovarian function as POF has been associated with hypoplasia of the thymus. In patients who have undergone ovarian biopsy as part of their evaluation, lymphocytic infiltration surrounding follicles has been described, as well as the resumption of menses after immunosuppression.
Immunoassays using antibodies directed at ovarian antigens have been developed and have demonstrated positive findings in some patients with POF, although the relevance of these findings remains unsettled. Ovarian autoantibodies could also conceivably be a secondary phenomenon to a primary cell-mediated form of immunity. Specific enzymes such as 3β-HSD may also be the target of ovarian autoimmunity. From a practical standpoint, screening for the common autoimmune disorders is appropriate in women found to have POF. Although relatively rare, as many as 2% to 4% of women with POF have antiadrenal antibodies, and may be at risk for adrenal failure. Commercial antibodies to 21-hydroxylase are available and agree with other assays for adrenal cortex antibodies. It has been suggested that screening women using dehydroepiandrosterone sulfate (DHEAS) also may be useful to detect signs of adrenal insufficiency.
More from a theoretical standpoint, abnormalities in the structure of gonadotropins, in their receptors, or in receptor binding could be associated with POF. While abnormal urinary forms of gonadotropins have been reported in women with POF, these data have not been replicated. Abnormalities of FSH-receptor binding, as mediated by a serum inhibitor, have been described. A genetic defect that may lead to alterations in FSH receptor structure was mentioned previously.
Under the category of ovarian insults, POF may be induced by ionizing radiation, chemotherapy, or overly aggressive ovarian surgery. Although not well documented, viral infections have been suggested to play a role, particularly mumps. A dose of 400 to 500 rads is known to cause ovarian failure 50% of the time, and older women are more vulnerable to experiencing permanent failure. A dose of approximately 800 rads is associated with failure in all women. Ovarian failure (transient or permanent) may be induced by chemotherapeutic agents, although younger women receiving this insult have a better prognosis. Alkalyzing agents, particularly cyclophosphamide, appear to be most toxic.
By exclusion, the majority of women are considered to have idiopathic POF because no demonstrable cause can be pinpointed. Among these women, small mutations in genes lying on the X chromosome, or yet to be identified autosomal genes, may be the cause.
Management of Primary Ovarian Failure/Insufficiency
Evaluation of women under age 30 who have POF should include screening for autoimmune disorders and a karyotype; detailed recommendations for screening of such women are available. In addition, vaginal ultrasound may be useful for assessing the size of the ovaries and the degree of follicular development, which, if present, may signify an immunological defect.
Treatment usually consists of estrogen replacement. If fertility is a concern, the most efficacious treatment is oocyte donation. Various attempts at ovarian stimulation are usually unsuccessful, and the sporadic pregnancies that may occur are just as likely to occur spontaneously (~5%) as with any intervention. These spontaneous pregnancies are frequently encountered while receiving estrogen replacement. Noting irregular or unscheduled bleeding (while receiving hormonal treatment [HT]) is important as a sign of endogenous sex steroid production. Serum AMH has been used to characterize women with ovarian insufficiency to determine where they may be in the spectrum of ovarian failure. However, AMH levels cannot help predict the chance of a spontaneous pregnancy. It has been shown in a large cohort of women ( n = 358) followed for POF, that spontaneous ovarian function may ensue in 24% of women, usually within a year of the diagnosis. In this longitudinal follow-up, 4.4% of women had spontaneous pregnancies.
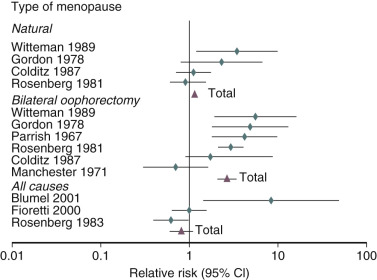
It has been well established that POF as well as bilateral oophorectomy prior to the usual age of menopause is associated with an increased risk of cardiovascular disease (CVD) as well as increased mortality ( Fig. 14.1 ). CVD mortality specifically is increased twofold to fourfold with early oophorectomy, and observational studies have suggested that using HT reduces this risk. A recent meta-analysis of women with POI suggested an increased risk of all-cause mortality, relative risk (RR): 1.39 (1.1 to 1.77). Early estrogen intervention has been shown to decrease all-cause mortality, which will be discussed later. Accordingly, unless there are contraindications, estrogen should be considered in all young women with POF, at least until the age of natural menopause.
Menopausal Transition (Perimenopause)
- ◆
Great variations in hormonal and menstrual findings occur around the final menstrual period.
- ◆
This is best described by STRAW+ 10, a workshop designed to describe these changes
- ◆
Prospective data have been generated by the Study of Women Across the Nation (SWAN) to describe the temporal changes of increasing FSH and decreasing estradiol around menopause.
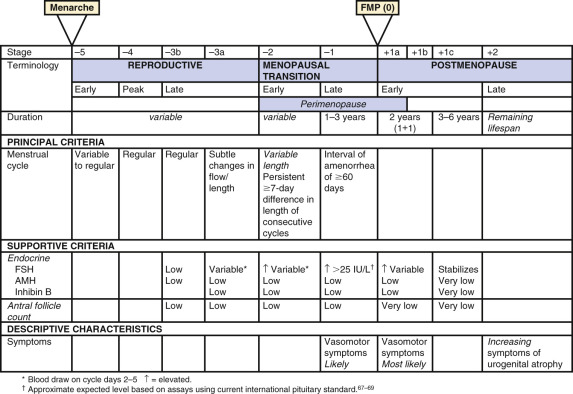
A workshop was convened in 2001 to build a consensus on describing various stages of the menopausal transition. Ten years later, another workshop was convened to update the staging system with more recent data. The Stages of Reproductive Aging Workshop + 10 (STRAW + 10) simplified bleeding criteria for the early and late menopausal transition and modified criteria for the late reproductive stage (stage −3) and the early postmenopausal stage (stage +1; Fig. 14.2 ). Then the late reproductive stages were expanded to include information on decreasing levels of AHM and inhibin B to reflect recent longitudinal data on fluctuating hormonal levels at the time of menopause, which will be described in more detail below.
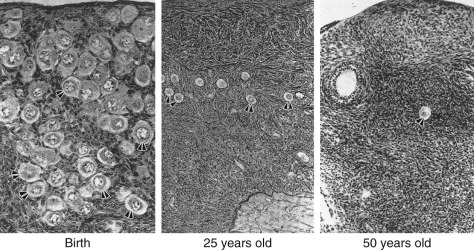
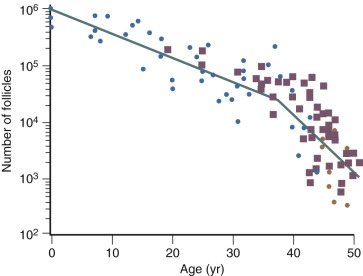
The ovary changes markedly from birth to the onset of menopause ( Fig. 14.3 ). The greatest number of primordial follicles is present in utero at 20 weeks’ gestation and undergoes a regular rate of atresia until around the age of 37. After this time, the decline in primordial follicles appears to become more rapid between age 37 and menopause ( Fig. 14.4 ) when no more than a thousand follicles remain. These remaining follicles are primarily atretic in nature.
Types of Ovarian Changes
Although perimenopausal changes are generally thought to be endocrine in nature and result in menstrual changes, a marked diminution of reproductive capacity precedes this period by several years. This decline may be referred to as gametogenic ovarian failure. The concept of dissociation in ovarian function is appropriate. Gametogenic failure is signified by reduced early follicular phase inhibin secretion, rising serum FSH levels, reduced antral follicle counts on ultrasound, decreased levels of AMH, and a marked reduction in fecundity. These changes may occur with normal menstrual function and no obvious endocrine deficiency, however, and they may occur in some women as early as age 35 (10 or more years before endocrine deficiency ensues).
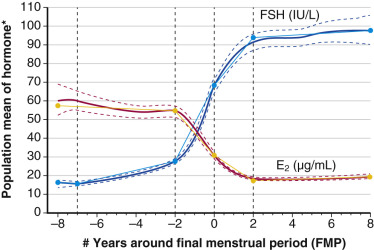
Recent longitudinal data obtained from the Study of Women Across the Nation (SWAN) have shown that estrogen levels begin to decline 2 years before the last menstrual period ( Fig. 14.5 ). Older data had shown that this only occurred during the last 6 months. The rise in FSH levels occurs several years before menopause but increases substantially in the last 2 years, and then stabilizes to steady-state levels 2 years after menopause. These study levels then decrease in the late menopause (seventh decade). There is also a very slow decline in androgen status (i.e., androstenedione and testosterone), which cannot be adequately detected at the time of the perimenopause.
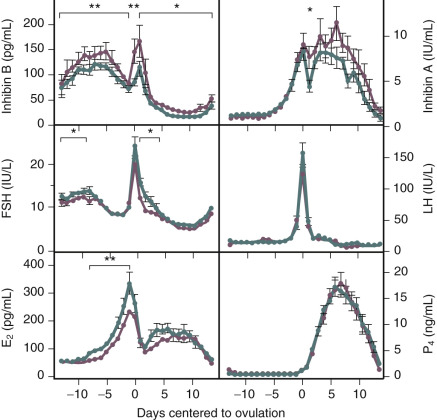
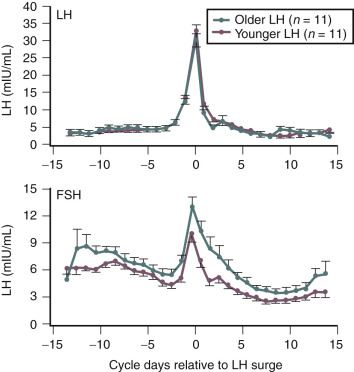
Products of the granulosa cell are most important for the feedback control of FSH. As the functional capacity of the follicular units decrease, secretion of substances that suppress FSH also decrease. Most notably, inhibin B levels are lower in the early follicular phase in women in their late 30s ( Fig. 14.6 ). Indeed, FSH levels are higher throughout the cycle in older ovulatory women than in younger women ( Fig. 14.7 ).
The functional capacity of the ovary is also diminished as women enter into the perimenopause. With gonadotropin stimulation, while estradiol (E 2 ) levels are not very different between younger and older women, total inhibin production by granulosa cells is decreased in women over age 35. From a clinical perspective, subtle increases in FSH on day 3 of the cycle, or increases in the clomiphene challenge test, correlate with decreased ovarian responses to stimulation and decreased fecundability.
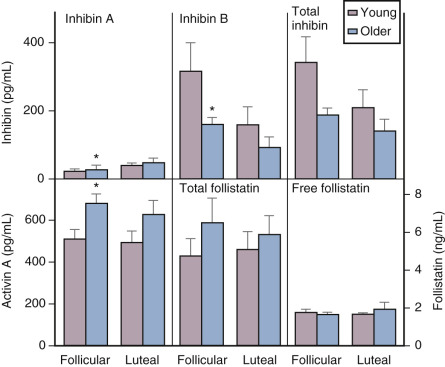
Although there is a general decline in oocyte number with age, an accelerated atresia occurs around age 37 or 38 (see Fig. 14.4 ). While the reason for this acceleration is not clear, one possible theory relates to activin secretion. Because granulosa cell-derived activin is important for stimulating FSH-receptor expression, the rise in FSH levels could result in more activin production, which, in turn, enhances FSH action. A profile of elevated activin with lower inhibin B has been found in older women ( Fig. 14.8 ). This autocrine action of activin, involving enhanced FSH action, might be expected to lead to accelerated growth and differentiation of granulosa cells. Further, activin has been shown to increase the size of the pool of preantral follicles in the rat. At the same time, these follicles become more atretic.
Clinical management of women in the perimenopause should address three general areas of concern: (1) irregular bleeding; (2) symptoms of menopause, such as hot flushes; and (3) the inability to conceive.
Treatment of irregular bleeding is complicated by the fluctuating hormonal status. Estrogen levels may be higher than normal in the early follicular phase and progesterone secretion may be normal, although not all cycles are ovulatory. For these reasons, short-term use of an oral contraceptive (usually 20 µg ethinyl estradiol) may be an option for otherwise healthy women who do not smoke to help them cope with irregular bleeding.
Early symptoms of menopause, particularly vasomotor changes, may occur as the result of fluctuating hormonal levels. In this setting, an oral contraceptive again may be an option if symptoms warrant therapy. Alternatively, lower doses of estrogen used alone may be another option.
Reproductive concerns often require more aggressive treatment because of decreased cycle fecundity. Once day 3 FSH levels increase and AMH levels are lower than the established normative data (usually less than 0.4 ng/mL), the prognosis for pregnancy is markedly reduced. A more detailed discussion of AMH levels may be found in the next section.
Hormonal Changes with Established Menopause
Depicted in Fig. 14.9 are the typical hormonal levels of postmenopausal women compared with those of ovulatory women in the early follicular phase. The most significant findings are the marked reductions in E 2 and estrone (E 1 ). Serum E 2 is reduced to a greater extent than E 1 . Serum E 1 , on the other hand, is produced primarily by peripheral aromatization from androgens, which decline more slowly, principally as a function of age. Levels of E 2 average 15 pg/mL and range from 10 to 25 pg/mL, but are 10 pg/mL or less in women who have undergone oophorectomy. Serum E 1 values average 30 pg/mL, but may be higher in obese women because aromatization increases as a function of the mass of adipose tissue.
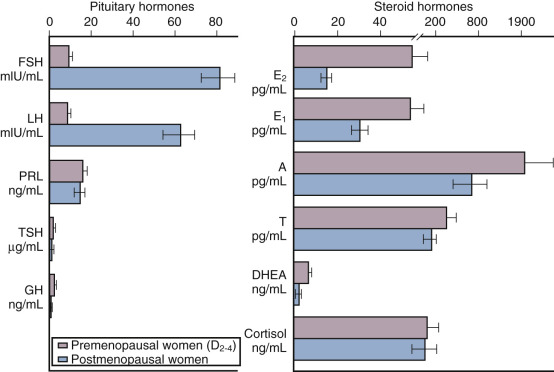
Estrone sulfate (E 1 S) is an estrogen conjugate that serves as a stable circulating reservoir of estrogen, and levels of E 1 S are the highest among estrogens in postmenopausal women. In premenopausal women, values are usually above 1000 pg/mL; in postmenopausal women, levels average 350 pg/mL.
Apart from elevations in FSH and luteinizing hormone (LH), other pituitary hormones are not affected. The rise in FSH, beginning in stage −3 as early as age 38 (see Fig. 14.2 ), fluctuates considerably until approximately 4 years after menopause when values are consistently greater than 20 mlU/mL. Specifically, growth hormone (GH), thyroid-stimulating hormone, and adrenocorticotropic hormone (ACTH) levels are normal. Serum prolactin levels may be very slightly decreased because prolactin levels are influenced by estrogen status.
Both the postmenopausal ovary and the adrenal gland continue to produce androgen. The ovary continues to produce androstenedione and testosterone but not E 2 , and this production has been shown to be at least partially dependent on LH. Androstenedione and testosterone levels are lower in women who have experienced bilateral oophorectomy, with values averaging 0.8 ng/mL and 0.1 ng/mL, respectively. The adrenal gland also continues to produce androstenedione, dehydroepiandrosterone (DHEA), and DHEAS; primarily as a function of aging, these values decrease somewhat (adrenopause), although cortisol secretion remains unaffected. Some data suggest that much “ovarian” testosterone production may actually arise from the adrenal. Most likely, this production is by indirect mechanisms due to the adrenal supplying precursor substrate (DHEA and androstenedione).
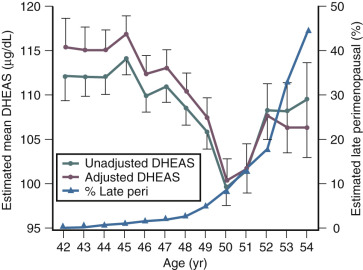
Although DHEAS levels decrease with age (approximately 2% per year), recent data have suggested that levels transiently rise in the perimenopause before the continuous decline thereafter ( Fig. 14.10 ). This interesting finding from the SWAN study also suggested that DHEAS levels are highest in Chinese women and lowest in African-American women.
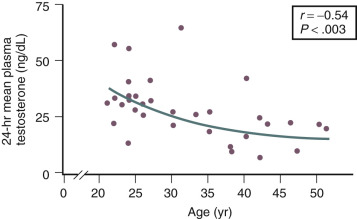
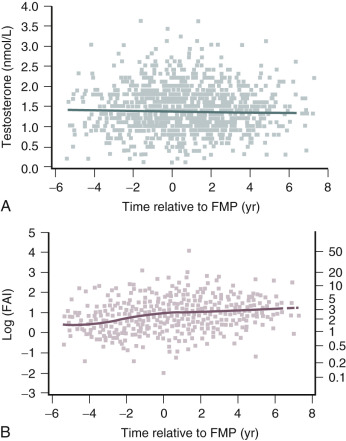
Testosterone levels also decline as a function of age, which is best demonstrated by the reduction in 24-hour mean levels ( Fig. 14.11 ). Because of the role of the adrenal in determining levels of testosterone after menopause, adrenalectomy or dexamethasone treatment results in undetectable levels of serum testosterone. Compared with total testosterone, the measurement of bioavailable or “free” testosterone is more useful in postmenopausal women. After menopause, sex hormone-binding globulin (SHBG) levels decrease, resulting in relatively higher levels of bioavailable testosterone or a higher free androgen index ( Fig. 14.12 ). In women receiving oral estrogen, bioavailable testosterone levels are extremely low because SHBG levels are increased. How this relates to the decision to begin androgen therapy in postmenopausal women will be discussed later in this chapter.
Elevated gonadotropin (FSH-LH) levels arise from reduced secretion of E 2 and inhibin as described earlier. Estrogen is important in controlling the production of gonadotropin-releasing hormone (GnRH) mRNA in type 1 neurons. In addition, the increase in gonadotropins observed at menopause appears to be enhanced by substance P, as well as by tachykinins produced in hypertrophied neurons, which result from the decrease in E 2 .
Unlike the rodent, where there is evidence for a hypothalamic factor involved in ovarian senescence, no such clear evidence exists for women. The hypothesis proposed by Wise suggested that the effects of aging in the brain affect neurotransmitter systems that regulate GnRH, disrupting ovarian folliculogenesis and ultimately promoting senescence. Thus the accelerated follicular loss that is apparent in the late 30s is postulated to be due to age-related desynchronization in the rhythmicity of GnRH secretion.
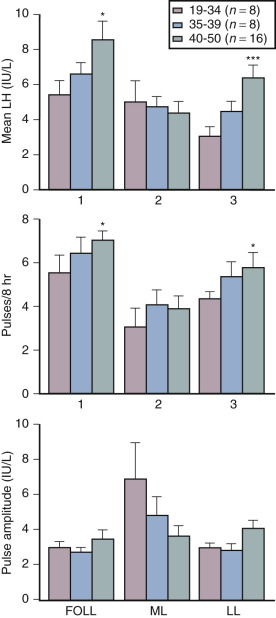
Although some aging effects of the brain are likely to exist, there is abundant human evidence for an ovarian-induced menopause. While there is slowing of LH pulsatility with aging in rodents, LH pulse frequency and amplitude increase with age as menopause approaches in women ( Fig. 14.13 ). A sleep-entrained alteration in GnRH pulse dynamics has been observed in postmenopausal women, namely the inability to increase GnRH pulse amplitude at night. There is some evidence that the high frequency and amplitude pulses of LH observed in the first few years in postmenopausal women slows down in late menopause ; this latter effect is clearly related to aging, per se.
Ovarian aging is a programmed event, and the return of atresia, accelerating at around age 37.5, until the natural age of menopause, has now been shown to occur in almost the exact way in the chimpanzee. Ovarian aging from a hormonal standpoint is best characterized by small elevations in serum FSH occurring at the beginning of the menstrual cycle (days 2 to 3), reductions in inhibin B, as well as steep declines in serum müllerian inhibiting substance or AMH was introduced previously ( Fig. 14.14 ). It has been confirmed that once a level of inhibin B or AMH becomes undetectable, menopause will ensue in 4 to 5 years (see Fig. 14.14A ). There have been several prediction models of the age of natural menopause based on levels of AMH. A recent meta-analysis showed that AMH has good predictability with a hazard ratio of 5.6 to 9.2. Nevertheless, there is no absolute precision with this prediction and it is questionable whether this knowledge has any practical value.
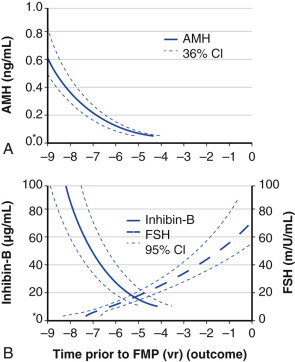
AMH is a very useful and practical determinant in that it tends to undergo less cycle-to-cycle variation compared to FSH or inhibin B, and can be measured during any phase of the cycle. However, it should be kept in mind that the use of oral contraceptive pills can reduce AMH levels by approximately 20%.
Effects on Various Organ Systems
- ◆
Estrogen has many powerful effects on brain anatomy and function, mediated through specific receptors.
- ◆
The hallmark feature of the drop in estrogen at the time of menopause is the hot flush or vasomotor instability.
- ◆
Recent data point to alterations in KNDy neurons in the hypothalamus at the time of menopause, which are linked to thermoregulatory centers in the brain.
- ◆
Collagen content decreases after menopause and is, at least in part, responsive to estrogen.
- ◆
Vulvovaginal complaints are common after menopause and increase with age, and are well defined.
- ◆
Treatment improves symptoms, including dyspareunia.
- ◆
Osteoporosis after menopause is occasioned by an increase in inflammatory factors that increase bone resorption in the face of estrogen decline.
- ◆
There are several biochemical and radiographic methods to assess bone turnover and bone density, but bone strength is most important in determining fracture risk.
- ◆
Many agents are now available for prevention and treatment.
- ◆
Degenerative arthritis correlates with aging, but it may also be related to estrogen insufficiency.
- ◆
CVD is the leading cause of death in women, and the risk increases from the onset of menopause.
- ◆
Estrogen protects against atherosclerosis prior to menopause and in younger women after menopause, before the development of established atherosclerosis.
- ◆
Estrogen treatment in younger healthy postmenopausal women decreases coronary disease and mortality but is ineffective in older women and may be harmful.
- ◆
Stroke risk in women increases with age and is compounded by obesity and hypertension.
- ◆
The risk in younger women is ischemic in nature and is mediated by thrombotic risk factors.
Central Nervous System
Estrogen has powerful effects on the brain mediated by estradiol receptors α and β as well as membrane effects. Most data point to neuroprotective effects of estrogen and older observational data point to a benefit of postmenopausal estrogen of cognitive decline and Alzheimer disease (AD), but this has not been confirmed in prospective studies. There are well-defined physiological and hormonal changes that occur during the hot flush—the hallmark feature of menopausal symptoms. A narrowing of the thermoregulatory zone is a theory for the etiology of the hot flush, but new data also point to alterations in hypothalamic kisspeptin, neurotensin, and dynorphin (KNDy) neurons at menopause.
The brain is an active site for estrogen action and estrogen formation. Estrogen activity in the brain is mediated via estrogen receptors (ER)α and ERβ receptors. Whether or not a novel membrane receptor (non-ERα/ERβ) exists is still being debated. However, both genomic and nongenomic mechanisms of estrogen action clearly exist in the brain. Fig. 14.15 illustrates the predominance of ERβ in the cortex (frontal and parietal) and the cerebellum, based on work in the rat. While 17β-E 2 is a specific ligand for both receptors, certain synthetic estrogens have a greater affinity for ERβ.
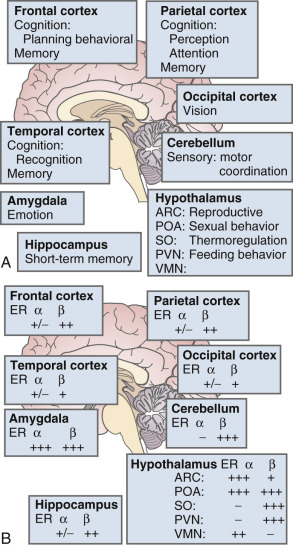
There are multiple actions of estrogen on the brain as reviewed by Henderson ( Box 14.2 ) ; thus, there are important functions linked to estrogen that contribute to well-being in general and, more specifically, to cognition and mood. The hallmark feature of declining estrogen status in the brain is the hot flush, which is more generically referred to as a vasomotor episode.
- 1.
Organizational actions
Effects on neuronal number, morphology, and connections occurring during critical stage of development
- 2.
Neurotrophic actions
Neuronal differentiation
Neurite extension
Synapse formation
Interactions with neurotrophins
- 3.
Neuroprotective actions
Protection against apoptosis
Antioxidant properties
Antiinflammatory properties
Augmentation of cerebral blood flow
Enhancement of glucose transport into the brain
Blunting of corticosteroid response to behavioral stress
Interactions with neurotrophins
- 4.
Effects on neurotransmitters
Acetylcholine
Noradrenaline
Serotonin
Dopamine
Glutamate
γ-Aminobutyric acid
Neuropeptides
- 5.
Effects on glial cells
- a.
Effects on proteins involved in Alzheimer disease
Amyloid precursor protein
Tau protein
Apolipoprotein E
- a.
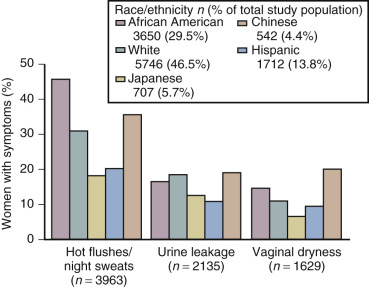
The hot flash usually refers to the acute sensation of heat, while the flush or vasomotor episode includes changes in the early perception of this event and other skin changes (including diaphoresis). Hot flushes usually occur for 2 years after the onset of estrogen deficiency, but can persist for 10 or more years. A recent longitudinal study from SWAN showed that the median duration of significant vasomotor symptoms is 7.4 years. In 10% to 15% of women, these symptoms are severe and disabling. In the United States, the incidence of these episodes varies in different ethnic groups. Symptoms are greatest in Hispanic and African-American women, intermediate in white women, and lowest among Asian women ( Fig. 14.16 ).
The fall in estrogen levels precipitate the vasomotor symptoms. Although the proximate cause of the flush remains elusive, the episodes result from a hypothalamic response (probably mediated by catecholamines) to the change in estrogen status. The flush has been well characterized physiologically. It results in heat dissipation as witnessed by an increase in peripheral temperature (finger, toe); a decrease in skin resistance, associated with diaphoresis; and a reduction in core body temperature ( Fig. 14.17 ). There are hormonal correlates of flush activity, such as an increase in serum LH and in plasma levels of pro-opiomelanocortin peptides (ACTH, β-endorphin) at the time of the flush, but these occurrences are thought to be epiphenomena and not the proximate cause of the flush. Nevertheless, as will be discussed below, there may be interplay in the hypothalamus between the thermoregulatory center and release of GnRH/LH.
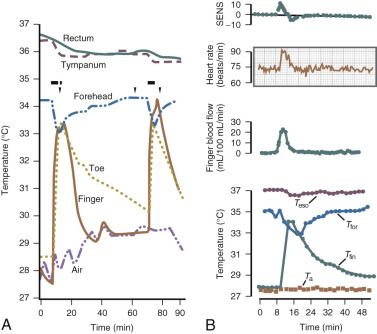
Data from Freedman have suggested that the major physiological finding in postmenopausal women with and without hot flushes is a narrowing of the temperature threshold for sweating and shivering in symptomatic women ( Fig. 14.18 ). This has been the major theory expounded for hot flushes occurring in an estrogen deficient state; however, recent evidence points to brain neuromodulatory changes. The KNDy neurons in the hypothalamus activate kisspeptin and other receptors on GnRH neurons and activation releases GnRH. At the same time, KNDy neurons also impinge upon the thermoregulatory center in the hypothalamus. Estrogen loss has been shown to increase the size of KNDy neurons, and to activate the genes for neurokinin B and kisspeptin as suggested in Fig. 14.19 . Experimentally, neurokinin B, activating neurokinin 3 has been shown to induce hot flushes in postmenopausal women. This activation of the KNDy neuronal system would also release GnRH/LH, which is what has been observed during a hot flush. The potential exists, therefore, that specific neurokinin (NK) antagonists (which are available for oral use) may be able to inhibit hot flushes in women.
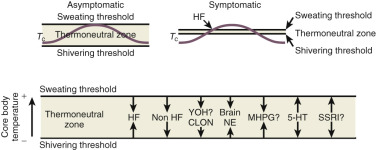

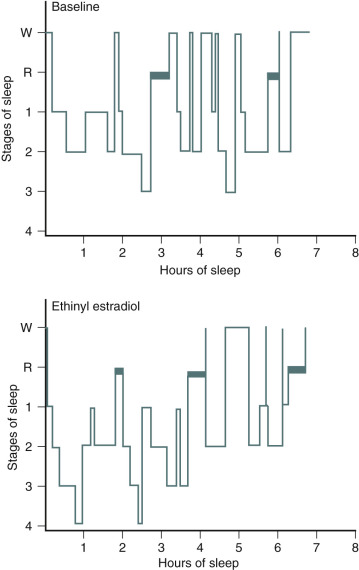
One of the primary complaints of women with hot flushes is sleep disruption. They may awaken several times during the night, and require a change of bedding and clothes because of diaphoresis. Nocturnal sleep disruption in postmenopausal women with hot flushes has been well documented by electroencephalographic (EEG) recordings. Sleep efficiency is lower, and the latency to rapid eye movement sleep is longer in women with hot flushes compared with asymptomatic women. This disturbed sleep often leads to fatigue and irritability during the day. The frequency of awakenings and of hot flushes are reduced appreciably with estrogen treatment ( Fig. 14.20 ). Sleep may be disrupted even if the woman is not conscious of being awakened from sleep. In this setting, EEG monitoring has indicated sleep disruption in concert with physiological measures of vasomotor episodes.
In postmenopausal women, estrogen has been found to improve depressed mood regardless of whether or not this is a specific complaint. (Critics of some of this work point out that mood is affected by the symptomatology and by sleep deprivation.) Blinded studies carried out in asymptomatic women have also shown benefit. In an estrogen-deficient state, such as occurs after the menopause, a higher incidence of depression (clinical or subclinical) is often manifest. However, menopause per se does not cause depression, and while estrogen does generally improve depressive mood, it should not be used for psychiatric disorders. Nevertheless, very high pharmacological doses of estrogen have been used to treat certain types of psychiatric depression in the past. Progestogens as a class generally attenuate the beneficial effects of estrogen on mood, although this effect is highly variable.
Cognitive decline in postmenopausal women is related to aging as well as to estrogen deficiency. The literature is somewhat mixed in showing whether there are benefits of estrogen in terms of cognition. In more recent studies, verbal memory appears to be enhanced with estrogen and has been found to correlate with acute changes in brain imaging, signifying brain activation.
Dementia increases as women age, and the most common form of dementia is AD. Listed in Box 14.2 are several neurotrophic and neuroprotective factors that relate to how estrogen deficiency may be expected to result in the loss of protection against the development of AD. In addition, estrogen has a positive role in enhancing neurotransmitter function, which is deficient in women with AD. This function of estrogen has particular importance and relevance for the cholinergic system that is affected in AD.
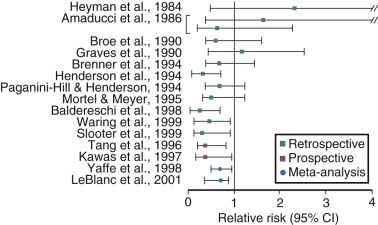
Estrogen use after menopause has been shown in observational studies to decrease the likelihood of developing or delay the onset of AD ( Fig. 14.21 ). However, there are no randomized prospective studies as yet on this issue. It is clear, however, that if estrogen has a beneficial role, it is only in those women who receive estrogen at the onset of menopause; in older women it may be detrimental. A recent short-term study could not show a cognitive benefit in women who received estrogen within 6 years of menopause and those who received it 10 years later. However, recent brain imaging studies have suggested that women treated early after menopause with estrogen may have less amyloid deposition, particularly if they have the Apo-E allele, a risk factor for AD. Once a woman is affected by AD, estrogen is unlikely to provide any benefit. In the Women’s Health Initiative (WHI) study, women over the age of 65 years receiving estrogen and progestogen for the first time had a decrease in cognition, which was not statistically significant for the group of women receiving estrogen alone. Still, to date, there are no compelling prospective data in younger postmenopausal women to confirm earlier observational studies on the benefits of estrogen in preventing cognitive decline or AD risk.
Collagen
Estrogen has a positive effect on collagen , which is an important component of bone and skin and serves as a major support tissue for the structures of the pelvis and urinary system. Both estrogen and androgen receptors have been identified in skin fibroblasts. Nearly 30% of skin collagen is lost within the first 5 years after menopause, and collagen decreases approximately 2% per year for the first 10 years after menopause. This statistic, which is similar to that of bone loss after menopause, strongly suggests a link between skin thickness, bone loss, and the risk of osteoporosis.
Although the literature is not entirely consistent, estrogen therapy generally improves collagen content after menopause and improves skin thickness substantially after about 2 years of treatment. There is a possible biomodal effect with high doses of estrogen causing a reduction in skin thickness. The supportive effect of estrogen on collagen has important implications for bone homeostasis and for the pelvis after menopause. Here, reductions in collagen support and atrophy of the vaginal and urethral mucosa have been implicated in a variety of symptoms, including prolapse and urinary incontinence.
Symptoms of urinary incontinence and irritative bladder symptoms occur in 20% to 40% of perimenopausal and postmenopausal women. Uterine prolapse and other gynecological symptoms related to poor collagen support, as well as urinary complaints, may improve with estrogen therapy. While estrogen generally improves symptoms, urodynamic changes have not been shown to be altered. Estrogen has also been shown to decrease the incidence of recurrence of urinary tract infections. These data relate to the use of vaginal estrogen, rather than the use of estrogen systemically. Somewhat paradoxically, systemic estrogen may increase stress urinary incontinence, while local vaginal therapy may improve urge incontinence.
In Sweden, the restoration of bladder control in older women with estrogen has been shown to decrease the need for admission to nursing homes. Estrogen may also have an important role in normal wound healing. In this setting, estrogen enhances the effects of growth factors, such as transforming growth factor β (TGFβ).
Genital Atrophy
Vulvovaginal complaints are often associated with estrogen deficiency. In the perimenopause, symptoms of dryness and atrophic changes occur in 21% and 15% of women, respectively. However, these findings increase with time, and by 4 years these incidences are 47% and 55%, respectively. With this change, an increase in sexual complaints also occurs, with an incidence of dyspareunia of 41% in sexually active 60-year-old women. Estrogen deficiency results in a thin and more pale vaginal mucosa. The moisture content is low, the pH increases (usually greater than 5), and the mucosa may exhibit inflammation and small petechiae.
With estrogen treatment, vaginal cytology changes have been documented, transforming from a cellular pattern of predominantly parabasal cells to one with an increased number of superficial cells. Along with this change, the vaginal pH decreases, vaginal blood flow increases, and the electropotential difference across the vaginal mucosa increases to that found in premenopausal women. Recent studies have suggested that intravaginal DHEA (0.25% to 1%) is efficacious for altering vaginal cytology and symptoms of atrophy, presumably by allowing for local conversion to other androgens and estrogen. This product (prasterone) is now commercially available, having been approved by the US Food and Drug Administration (FDA) for symptoms of dyspareunia in postmenopausal women.
Bone Loss
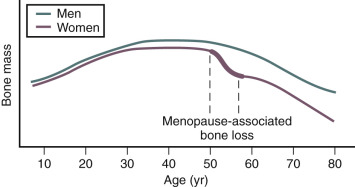
Estrogen deficiency has been well established as a cause of bone loss. This loss can be noted for the first time when menstrual cycles become irregular in the perimenopause. From 1.5 years before the menopause to 1.5 years after menopause, spine bone mineral density (BMD) has been shown to decrease by 2.5% per year, compared with a premenopausal loss rate of 0.13% per year. Loss of trabecula bone (spine) is greater with estrogen deficiency than is loss of cortical bone.
Postmenopausal bone loss leading to osteoporosis is a substantial healthcare problem. In white women, 35% of all postmenopausal women have been estimated to have osteoporosis based on BMD. Further, the lifetime fracture risk for these women is 40%. The morbidity and economic burden of osteoporosis is well documented. Interestingly, there are data to suggest that up to 19% of Caucasian men also have osteoporosis.
Bone mass is substantially affected by sex steroids through classic mechanisms to be described later in this chapter. Attainment of peak bone mass in the late second decade ( Fig. 14.22 ) is key to ensuring that the subsequent loss of bone mass with aging and estrogen deficiency does not lead to early osteoporosis. Estradiol, together with GH and insulin-like growth factor-1, acts to double bone mass at the time of puberty, beginning the process of attaining peak bone mass. Postpubertal estrogen deficiency (amenorrhea from various causes) substantially jeopardizes peak bone mass. Adequate nutrition and calcium intake are also key determinants. While estrogen is of predominant importance for bone mass in both women and men, testosterone is important in stimulating periosteal apposition; as a result, cortical bone in men is larger and thicker.
ERs are present in osteoblasts, osteoclasts, and osteocystes. Both ERα and ERβ are present in cortical bone, while ERβ predominates in cancellous or trabecular bone. However, the more important actions of estradiol are believed to be mediated via ERα.
Estrogens suppress bone turnover and maintain a certain rate of bone formation. Bone is remodeled in functional units, called bone multicenter units (BMUs), where resorption and formation should be in balance. Multiple sites of bone go through this turnover process over time. Estrogen decreases osteoclasts by increasing apoptosis and thus reduces their lifespan. The effect on the osteoblast is less consistent, but E 2 antagonizes glucocorticoid-induced osteoblast apoptosis. Estrogen deficiency increases the activities of remodeling units, prolongs resorption, and shortens the phase of bone formation ; it also increases osteoclast recruitment in BMUs, thus resorption outstrips formation.
The molecular mechanisms of estrogen action on bone involve the inhibition of production of proinflammatory cytokines including interleukin-1, interleukin-6, and tumor necrosis factor, which inhibits bone resorption. Receptor-activation of nuclear factor kappa-B ligand (RANKL) is responsible for osteoclast differentiation and action. A scheme for how all these factors interact has been proposed by Riggs ( Fig. 14.23 ).
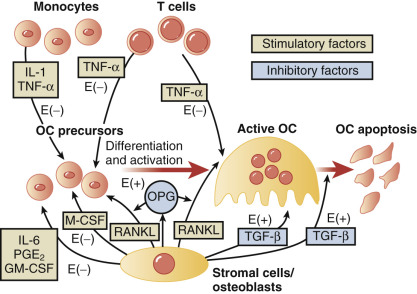
In women, Riggs has suggested that bone loss occurs in two phases. In the first phase, with declining estrogen at the onset of menopause there is an accelerated phase of bone loss; this loss is predominantly of cancellous or trabecula bone. Here 20% to 30% of cancellous bone and 5% to 10% of cortical bone can be lost in a short span of 4 to 8 years. Thereafter, a slower phase of loss (1% to 2% per year) ensues where, proportionately, more cortical bone is lost. This phase is thought to be induced primarily by secondary hyperparathyroidism. The first phase is also accentuated by the decreased influence of stretching or mechanical factors, which generally promote bone homeostasis, as a result of estrogen deficiency.
Genetic influences on bone mass are more important for attainment of peak bone mass (heritable component, 50% to 70%) than for bone loss. Polymorphisms of the vitamin D receptor gene, TGFβ gene, and the Spl-binding site in the collagen type 1 AI gene have all been implicated as being important for bone mass.
While testosterone is important for bone formation and stimulation of bone mass, even in men estrogen action is of paramount importance. Bone mass was shown to increase in an aromatase-deficient man upon estrogen administration.
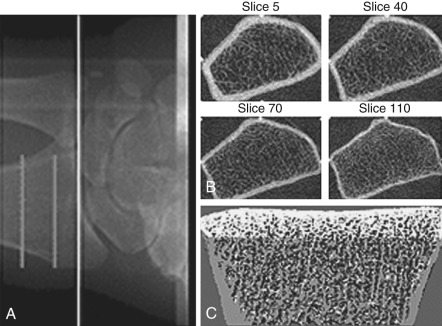
Bone mass can be detected by a variety of radiographic methods ( Table 14.2 ). Dual energy x-ray absorptiometry scans have become the standard of care for detection of osteopenia and osteoporosis. By convention, the T score is used to reflect the number of standard deviations of bone loss from the peak bone mass of a young adult. Osteopenia is defined by a T score of −1 to −2.5 standard deviations; osteoporosis is defined as greater than 2.5 standard deviations. Since bone mass does not completely reflect bone strength, which is really what matters in terms of fracture risk, several approaches have been made to assess bone strength. An assessment of biochemical bone turnover (discussed later in the chapter), in addition to bone mass, is deemed important. One newer approach is to assess bone microarchitecture, a so-called virtual bone biopsy, by using a high-resolution (HR) peripheral (p) quantitative computed tomography ( Fig. 14.24 ). This technique has been used to assess bone strength and formation in boys and girls to assess sex differences in bone growth and strength, and the risk of fracture. Various biochemical assays are also available to assess bone resorption and formation in both blood and urine ( Table 14.3 ). At present, serum markers appear to be most useful for assessing changes with antiresorptive therapy. Biochemical assays can provide some functional information, which may be helpful in assessing bone strength, and can reflect changes in bone resorption/formation more rapidly than imaging studies. However, these assays do not correlate well with bone density measurements.
| Technique | Anatomical Site of Interest | Precision in vivo (%) | Examination and Analysis Time (min) | Estimated Effective Dose Equivalent (µSv) |
|---|---|---|---|---|
| Conventional radiography | Spine, hip | NA | <5 | 2000 |
| Radiogrammetry | Hand | 1–3 | 5–10 | <1 |
| Radiographic absorptiometry | Hand | 1–2 | 5–10 | <1 |
| Single x-ray absorptiometry | Forearm, heel | 1–2 | 5–10 | <1 |
| Dual x-ray absorptiometry | Spine, hip, forearm, total body | 1–3 | 5–20 | 1–10 |
| Quantitative computed tomography | Spine, forearm, hip | 2–4 | 10–15 | 50–100 |
| Quantitative ultrasound | Heel, hand, lower leg | 1–3 | 5–10 | None |
| Marker | Specimen |
|---|---|
| Bone Resorption Markers | |
| Cross-linked N-telopeptide of type 1 collagen (NTX) | Urine, serum |
| Cross-linked C-telopeptide of type 1 collagen (CTX) | Urine (αα and ββ forms) |
| Serum (ββ form) | |
| MMP-generated telopeptide of type 1 collagen (ICTP or CTX-MMP) | Serum |
| Deoxypyridinoline, free and peptide bound (fDPD, DPD) | Urine, serum |
| Pyridinoline, free and peptide bound (fPYD, PYD) | Urine, serum |
| OHP | Urine |
| GylHyl | Urine, serum |
| HelP | Urine |
| Tartrate-resistant acid phospharase | Serum, plasma |
| 5b Isoform specific for osteoclasts (TRACP 5b) | |
| Cath K | Urine, serum |
| uOC | Urine |
| Bone Formation Markers | |
| OC | Serum |
| Procollagen type 1 C-terminal propeptide (PICP) | Serum |
| Procollagen type 1 N-terminal propeptide (PINP) | Serum |
| Bone-specific alkaline phosphatase (bone ALP) | Serum |
There are now many agents that can prevent osteoporosis. The use of estrogen will depend on whether or not there are other indications for estrogen treatment and whether there are contraindications. Estrogen has been shown to reduce the risk of osteoporosis as well as to reduce osteoporotic fractures. In the WHI study, hip fractures, as well as all fractures, were reduced with conjugated equine estrogens (CEE) and medroxyprogesterone acetate (MPA), and CEE alone, and this occurred in a nonosteoporotic population. Indeed, in a cohort of women who were followed after stopping hormones when the results of the WHI study were published, there was an increased risk of hip fracture and lower bone density compared to those women who continued therapy.
A dose equivalent to 0.625 mg of CEE was once thought to prevent osteoporosis, but we now know that lower doses (0.3 mg of CEE or its equivalent) in combination with progestogens are able to prevent bone loss, although there are no data on fractures. Whether or not the addition of progestogens, by stimulating bone formation, increases bone mass over that of estrogen alone is unclear. The androgenic activity of certain progestogens such as northindrone acetate also has been suggested to play a role.
Selective estrogen receptor modulators (SERMs) , such as raloxifene, droloxifene, and tamoxifen, have all been shown to decrease bone resorption. Raloxifene has been shown to decrease vertebral fractures in a large prospective trial. Tibolone has also been shown to be an effective treatment for osteoporosis. Tibolone (not marketed in the United States) has SERM-like properties, but it is not specifically a SERM because it has mixed estrogenic, antiestrogenic, androgenic, and progestogenic properties due to its metabolites. The drug does not cause uterine or breast cell proliferation and is beneficial for vasomotor symptoms. It prevents osteoporosis and has been shown to be beneficial in the treatment of established osteoporosis.
Bisphosphonates have been shown to have a significant effect on the prevention and treatment of osteoporosis. With this class of agents (etidronate, alendronate, residronate, ibandronate, and zoledronic acid) there is incorporation of the bisphosphonate with hydroxyapetite in bone, which increases bone mass. The skeletal half-life of bisphosphonates in bone can be as long as 10 years. Most data have been derived with alendronate, which, at a dosage of 5 mg daily (35 mg weekly) prevents bone loss; at 10 mg daily (70 mg weekly), alendronate is an effective treatment for osteoporosis, with evidence available that this treatment reduces vertebral and hip fractures. Ibandronate is available as a monthly pill (150 mg) and by injection (3 mg) every 3 months. It has primary efficiency for vertebral fracture protection. Zoledronic acid, 5 mg, is available as an intravenous therapy, with infusion over 15 minutes.
There has been some concern over this class of very powerful bone resorption agents causing fractures of the long bones such as the femur because of “brittle” bone. This only occurs with prolonged use of at least 7 years, and the incidence is in the range of 3.2 to 50 per 100,000 women. Osteonecrosis of the jaw has also been cited as a concern, but this mainly occurs in the presence of poor dental health, and is rare with an incidence in the range of 1/10,000 women. With long-term therapy, atrial fibrillation, as an adverse event also occurs, although this too is rare. These adverse effects appear to be a “class” effect of bisphosphonates. Nevertheless, while all these findings are rare events, there are no good data for prolonged treatment of 10 or more years. For these and other reasons, bisphosphonates are not the drugs of choice in younger women prior to natural menopause and should not be used in women who wish to conceive.
Calcitonin, 50 IU subcutaneous injections daily or 200 IU intranasally, has been shown to inhibit bone resorption. Vertebral fractures have been shown to decrease with calcitonin therapy. However, long-term effects have not been established.
Fluoride has been used for women with osteoporosis because it increases bone density. Currently, a lower dose (50 µg daily) of slow-release sodium fluoride does not seem to cause adverse effects (gastritis) and has efficacy in preventing vertebral fractures.
Denosumab is a monoclonal antibody targeting RANKL, which is secreted by osteoblasts and causes bone resorption (see Fig. 14.22 ). Thus it is an antiresorptive agent with significant potency. Denosumab 60 mg is administered subcutaneously every 6 months, and while it has a preventative role it is usually considered to be a second-line treatment for difficult-to-treat cases. Denosumab has efficacy both at the vertebrae and the hip, but unlike the bisphosphonates, is shorter acting and wears off quickly rather than being bound to the bone with a long half-life in the case of bisphosphonates. Also it is devoid of the other side effects of bisphosphonates (fractures, osteonecrosis, etc.) described above. Because of this profile and its benefit at the hip as well as the spine, denosumab has emerged as a popular therapy. Nevertheless, as a newer form of immune therapy, long-term potential consequences are not known.
An inhibitor of cathepsin K, odanacatib, also has a significant effect on decreasing bone resorption and has been successful in clinical trials at vertebral and nonvertebral bone sites. However, odanacatib is not yet available for clinical use.
Intermittent parathyroid hormone (PTH 1-34, teriparatide) is an agent that increases bone mass in women with osteoporosis. In a randomized trial lasting 3 years, average bone density increased in the hip and spine with fewer fractures observed. This therapy is available in the United States, at a standard dose of 20 µg/day injected subcutaneously for no longer than 18 months. It is expensive and is reserved for women who are difficult to treat and have a history of fractures.
Another agent, which has the ability to increase bone formation, is a monoclonal antibody against sclerosin. Sclerosin is an inhibitor of normal bone formation, which activated the Wnt signaling system. While this monoclonal antibody, romosozumab, has shown great efficacy in increasing bone formation, its cardiovascular risk profile has not been acceptable; and it is unlikely that this drug will be available for clinical use.
Adjunctive measures for the prevention of osteoporosis are calcium, vitamin D, and exercise. Calcium with vitamin D treatment has been shown to increase bone only in older individuals. These modalities alone are not thought to be effective for the treatment of osteoporosis. A woman’s total intake of elemental calcium should be 1200 to 1500 mg daily if no agents are being used to inhibit resorption, 400 to 800 IU of vitamin D should also be ingested. Levels of serum 25 hydroxy vitamin D have been found to be abnormally low (<20 ng/mL) in a large population of women, particularly in geographic regions of less sunlight exposure. Exercise has been shown to be beneficial for building muscle and bone mass and for reducing falls. Guidelines regarding the management of osteoporosis were published by the National Osteoporosis Foundation, last updated in 2013 ( www.nof.org ). An update analysis has suggested that 37% of women are candidates for treatment to prevent fractures. Also, the World Health Organization (WHO) has produced guidelines for assessing an individual’s risk of osteoporosis based on history, anthropometry, and BMD. This new paradigm, called the Fracture Risk Assessment Tool, may be obtained at www.shef.ac.uk/FRAX .
Degenerative Arthritis
Chronic arthritis is a major source of disability in postmenopausal women, and while this is largely correlated with older age, there is some evidence that estrogen deficiency after menopause may contribute to its progression. Administration of estrogen after menopause has been shown to inhibit the damage to chondrocytes, which is a key activator of the problem of arthritis. In the WHI study, the estrogen alone trial showed evidence for a significant decrease in osteoarthirits. Nevertheless, much more work is needed in this area.
Cardiovascular Effects
Clearly after menopause, the risk of CVD in women is increased. Data from the Framingham study have shown that the incidence is three times lower in women before menopause than in men (3.1 per 1000 per year in women aged 45 to 49). The incidence is approximately equal in men and women aged 75 to 79 (53 and 50.4 per 1000 per year, respectively). This trend also pertains to gender differences in mortality due to CVD. Coronary artery disease is the leading cause of death in women, and the lifetime risk of death is 31% in postmenopausal women versus a 3% risk of dying of breast cancer.
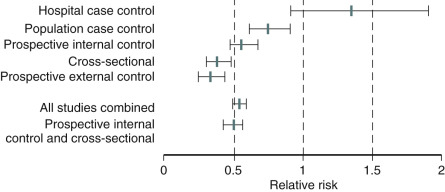
Although CVD becomes more prevalent only in the later years following a natural menopause, premature cessation of ovarian function (before the average age of menopause) constitutes a significant risk. Premature menopause, occurring before age 35, has been shown to increase the risk of myocardial infarction twofold to threefold, and oophorectomy before age 35 increases the risk severalfold.
An analysis of several studies on this issue has been conducted and reviewed, as shown in the data depicted in Fig. 14.25 , on the effect of early menopause on the prevalence and type of CVD. It has been suggested that total mortality is increased if bilateral oophorectomy occurs even after the natural menopause, until around age 60. This change in total mortality is due to an excess in coronary disease, suggesting a protective effect of the ovary even beyond the normal age of menopause.
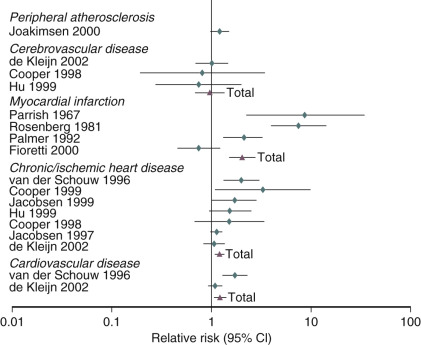
When the possible reasons for the increase in CVD are examined, the most prevalent finding is that of the accelerated rise in total cholesterol in postmenopausal women. The changes of weight, blood pressure, and blood glucose with aging, while important, are not thought to be as important as the rate of rise in total cholesterol, which is substantially different in women versus men. This increase in total cholesterol is explained by increases in levels of low-density lipoprotein cholesterol (LDL-C). The oxidation of LDL-C is also enhanced, as are levels of very-low-density lipoproteins and Lp(a) lipoprotein. HDL-C levels trend downward with time, but these changes are small and inconsistent relative to the increases in LDL-C.
Coagulation balance is not substantially altered as a counterbalancing change occurs. Some procoagulation factors increase (Factor VII, fibrinogen, and plasminogen activator inhibitor-1 [PAI-1]), but so do counterbalancing factors like antithrombin III, plasminogen, protein C, and protein S. Inflammation markers, such as C-reactive protein and cytokines, are increased; and blood flow in all vascular beds decreases after menopause. Prostacyclin production decreases, endothelin levels increase, and vasomotor responses to acetylcholine are constrictive, reflecting reduced nitric oxide synthetase activity. With estrogen, all these parameters (generally) improve and coronary arterial responses to acetylcholine are dilatory with a commensurate increase in blood flow. Circulating plasma nitrites and nitrates have also been shown to increase with estrogen, and angiotensin-converting enzyme levels tend to decrease. Estrogen and progesterone receptors have been found in vascular tissues, including coronary arteries (predominantly ERβ). In addition, there are membrane effects mediated by estrogen—which may or may not relate to either ERα or ERβ. The statements above pertain to the effects of estrogen in a relatively normal coronary artery (devoid of significant atherosclerosis). Once there is significant atherosclerotic plaque, the normal actions of estrogen do not occur ( Fig. 14.26 ).
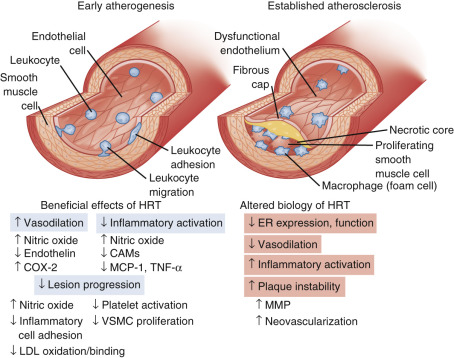
Overall, the direct vascular effects of estrogen are viewed to be as important, or more important, than the changes in lipid and lipoproteins after menopause. While replacing estrogen has been thought to be beneficial for the mechanisms previously cited, these beneficial arterial effects can only be seen in younger (stage +1) postmenopausal women. Women with significant atherosclerosis or risk factors, such as those studied in a secondary prevention trial, do not respond in a beneficial manner (see Fig. 14.26 ). Some of this lack of effect may be accounted for by increased methylation of the promoter region of ERα, which occurs with atherosclerosis and aging.
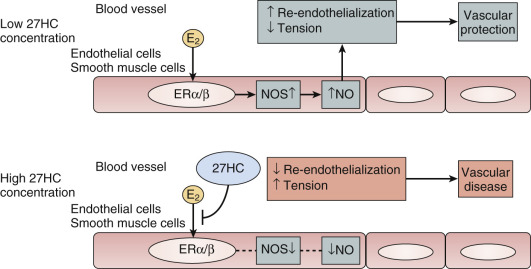
Another theory proposed to explain differences in the effects of estrogen when given earlier rather than later is the interfering effect of endogenous 27-hydroxy cholesterol. This endogenous metabolite of cholesterol increases with advancing levels of cholesterol and competes for binding with E 2 at the ER in the endothelium. Thus, when cholesterol is elevated, high levels of 27-C may prevent estrogen action ( Fig. 14.27 ).
In normal, nonobese postmenopausal women, carbohydrate tolerance also decreases as a result of an increase in insulin resistance. This, too, may be partially reversed by estrogen. In postmenopausal women, use of hormones decreases the risk of new onset diabetes.
Biophysical and neurohormonal responses to stress (stress reactivity) are exaggerated in postmenopausal women compared with premenopausal women, and this heightened reactivity is blunted by estrogen. Whether or not these changes influence cardiovascular risk with estrogen deficiency is not known, but clearly estrogen treatment returns many parameters into the range of premenopausal women in early postmenopausal women.
These consistently strong basic science and clinical data for the protective effects of estrogen on the cardiovascular system, together with strong epidemiological evidence for a protective effect of estrogen ( Fig. 14.28 ), led to the belief that estrogen should be prescribed to prevent CVD in women. However, randomized clinical trials (RCTs) in women with established disease did not find benefit, and there was a trend towards more harm. Results from several “secondary prevention” randomized trials in women who had coronary disease, found a lack of benefit, and in some studies, there were more coronary events in older women given estrogen at a standard dose for the first time.
This trend toward increased cardiovascular events in women who are older and/or who have documented diseased coronary arteries (“early harm”) occurs within the first 1 to 2 years. This is thought to result from oral estrogen increasing circulating levels of matrix metalloproteinases, which dissolve part of the gelatinous plaque in the mural portion of an atherosclerotic coronary vessel, causing plaque instability and rupture resulting in a coronary thrombosis. The WHI trial, which compared CEE/MPA with placebo, showed this effect in older women. This trial was considered to be a primary prevention trial, but with a mean age of 63 years and a range up to 79 years, there were many fewer younger women in their 50s recruited, and the higher frequency of cardiovascular events reported for the entire study was predominantly explained by the findings in the older cohort of women.
The protective effect of estrogen demonstrated in the Nurse’s Health Study (see Fig. 14.28 ) as well as other observational cohorts, evaluated by meta-analyses, was because treatment was carried out in predominantly young healthy women who were symptomatic, and were receiving estrogen for menopausal symptoms.
Table 14.4 compares the demographics of the participants of WHI and the Nurse’s study. Trials carried out in the monkey model have shown that there is a 50% to 70% protective effect against coronary atherosclerosis when estrogen is begun at the time of oophorectomy, with or without an atherogenic diet; delaying the initiation of hormonal therapy for even 2 years (in the monkey) prevents this protective effect ( Fig. 14.29 ). This early intervention is thought to relate to the first 6 years after menopause in women.
| Characteristic | NHS | WHI |
|---|---|---|
| Mean age or age range at enrollment (years) | 30–55 | 63 |
| Smokers (past and current) | 55% | 49.9% |
| BMI (mean) | 25.1 kg/m 2 | 28.5 kg/m 2 * |
| Aspirin users | 43.9% | 19.1% |
| HT regimen | Unopposed or sequential | Continuous-combined |
| Menopausal symptoms (flushing) | Predominant | Uncommon |

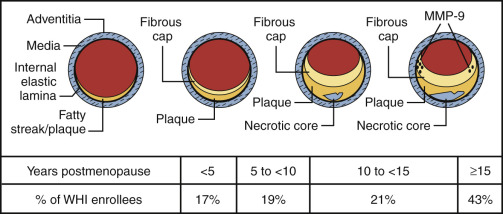
It is now well established that the late treatment of postmenopausal women with standard doses of estrogen may be harmful and affords no coronary protection. This finding pertains to various trials with end points of coronary events, or angiographically determined disease, as noted above, and does not appear to be modified by the hormonal regimen or route of administration. This is also true whether or not the woman has sustained a known coronary event. Since atherosclerosis is highly age dependent, even women who have not had a coronary event have diseased arteries ( Fig. 14.30 ). Also, as shown in Fig. 14.30 , well over 70% of the women studied in the WHI would have been expected to have atherosclerotic vessels.
As noted previously, the “early harm” observed when older women are exposed to estrogen for the first time is likely due to plaque instability and rupture. It has been reported that these effects in older women were not observed in those women receiving statins concurrently. Statins are known to stabilize plaque. In women who had been receiving estrogen for a prolonged time, mortality was decreased in those who sustained a myocardial infarction. Young women initiating hormones within the first few years of menopause do not appear to have the risk described in previous text. No increase in cardiovascular events was found in young healthy symptomatic women during the first 2 years of various hormonal regimens in clinical trials.
In concert with the view that estrogen given to early (and younger) postmenopausal women may have different effects, data have been analyzed in the 50- to 59-year-old age group in WHI, and in those less than 10 years from menopause. The definitions of menopause in WHI were not precise, and more women in the 50- to 59-year-old age group were older than 55 years old. The data are in strong contrast to the results of the entire group (two-thirds over 60 years old). In the estrogen only arm of WHI (hysterectomized women) receiving CEE 0.625 mg, the 50- to 59-year-old age group had reduced coronary event scores of borderline significance and a composite coronary score of statistical significance. An analysis of 20 RCTs in younger women (which included WHI) showed a statistically significant benefit in the reduction of coronary events and mortality. Younger women in WHI using estrogen only also had significantly reduced coronary calcium. Ten-year follow up data of the estrogen alone arm published in 2011 confirmed that the 50- to 59-year-old group had a significant reduction in coronary heart disease events and total mortality. The latest long-term follow-up of both the estrogen alone and the estrogen/progestogen arms of the hormone trials, was published in 2013 ( Fig. 14.31 ). Clearly younger women, aged 50 to 59 or less than 10 years from menopause, had a much better risk-benefit profile compared to older women. The effects of estrogen alone may be summarized in Table 14.5 . However, it should be noted that even with CEE/MPA, with 13 years of follow-up data, the coronary events witnessed were not increased for the entire group or even in any specific age group, when considered separately. This is in vast contrast to the original publication in 2002, which suggested a significantly increased coronary risk in all groups.
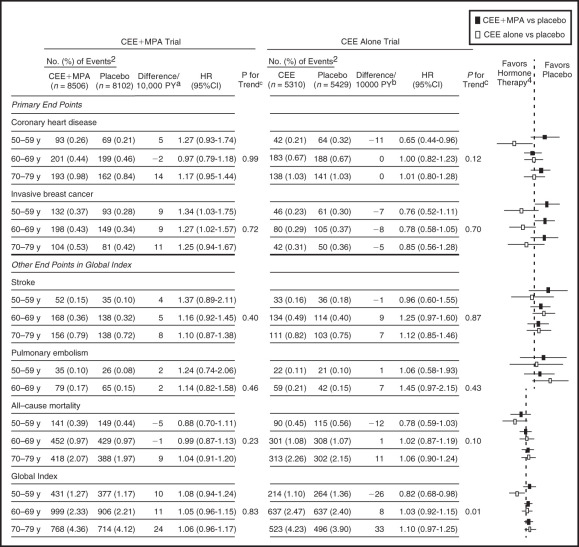
| Coronary heart disease | 0.65 (0.44–0.96) | −11/10,000 woman years |
| Myocardial infarction | 0.60 (0.39–0.91) | −11/10,000 woman years |
| Breast cancer | 0.76 (0.52–1.11) | −7/10,000 woman years |
| All cancers | 0.80 (0.64–0.99) | −18/10,000 woman years |
| Global index | 0.82 (0.82–0.98) | −26/10,000 woman years |
| Mortality | 0.78 (0.59–1.03) | −12/10,000 woman years |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree