Melanoma
EPIDEMIOLOGY, RISK FACTORS, SCREENING, AND PREVENTION
 EPIDEMIOLOGY
EPIDEMIOLOGY
The incidence of melanoma has dramatically risen over the past several decades and in 2013, an estimated 76,690 new cases and 9,480 deaths were expected in the United States (1). As a result, melanoma is now the fifth and sixth most common malignancy in the United States among men and women, respectively (1).
 RISK FACTORS
RISK FACTORS
Risk factors for melanoma include light complexion, poorly tanning skin, and blonde or red hair, which confer a relative risk of 1.3–4.1 (2). Intense, intermittent sun exposure and a history of blistering sunburn appear to confer a greater risk than lower-level, continuous sunlight exposure (3). Both ultraviolet A (UVA) and ultraviolet B (UVB) radiation have been implicated in the pathogenesis of melanoma (4). Exposure to UV radiation via tanning booths and, with psoralen, as a treatment for psoriasis, is associated with an increase in the risk of melanoma (5).
Numerous common nevi are a marker of increased risk, as are atypical nevi (6). Large congenital nevi have a high risk of malignant transformation (lifetime risk 4%–10%) (7–9). Following the diagnosis of melanoma, the probability of developing a second primary melanoma has been estimated to be 5.34% over an interval of 20 years (10).
Melanoma in a first-degree relative confers an increased risk, and about 10% of individuals diagnosed with melanoma have an affected family member (11, 12). However, the magnitude of the risk associated with family history is quite variable. Most families with multiple affected members have no identifiable genetic abnormality. Genes in which germline mutations or polymorphisms have been associated with an increased risk of melanoma include CDKN2A and CDK4, which encode cell cycle regulatory proteins, the melanocortin 1 receptor gene, and the breast cancer susceptibility gene, BRCA2 (relative risk for melanoma, 2.58) (13–16).
The dysplastic nevus syndrome is characterized by numerous atypical nevi and the development of melanoma at an early age (17). The lifetime risk of melanoma approaches 100% in this syndrome. Its genetic basis remains unknown.
 SCREENING
SCREENING
The majority of melanomas display at least one of the following features:
A: Asymmetry
B: Irregularity of borders
C: Color variegation
D: Diameter >6 mm
E: Enlargement or evolution
Ten percent of incident cases of melanoma are classified as having nodular histology and, therefore, lack the characteristic asymmetry, irregularity of border, and color variegation of the more common superficial spreading melanoma. However, nodular melanomas are associated with the poorest survival of all subtypes and account for a disproportionate number of melanoma deaths (18).
Melanoma is highly curable by surgical excision when detected at an early stage (i.e., less than 1 mm in thickness), whereas the risk of mortality rises sharply with thicker lesions (19). The American Academy of Dermatology has sponsored a screening program for over one million individuals. In a subgroup for which pathologic data were available, a presumptive diagnosis of melanoma was made in 0.8% and confirmed in 0.15%. The highest yield was among white males over the age of 50 years, a population that also has the highest risk for mortality from melanoma (20). While no survival data are available from this program, it lends support to the feasibility, and possibly the efficacy, of large-scale screening, as do similar programs in other countries. The overall benefit and cost-effectiveness of screening remain to be determined.
 PREVENTION
PREVENTION
The efficacy of topical sunscreens in the primary prevention of melanoma has not been rigorously demonstrated (21). Effective melanoma prevention strategies will probably involve a combination of education and behavior modification beginning at an early age. Such programs are underway in the United States, Australia, and other endemic areas, but their impact is difficult to gauge at this point.
For decades, although there was a clear link between sun-exposure or recreational exposure to UV radiation (i.e., tanning beds) and melanoma, no definitive, randomized evidence had existed which proved that sun-screen use prevents melanomagenesis (3, 4). It was thought that this may have been be due to the fact that commercially available sunscreens reduce exposure to UVB radiation but not to UVA, that they were inadequately applied, or that they provided a false sense of security leading to more prolonged sun exposure. The first randomized trial rigorously evaluating the use of sunscreen for the prevention of melanoma was reported in 2011 (22). In this study, investigators randomized 1621 participants in Nambour, Australia (ages 25–75 years) to either sunscreen intervention or no sunscreen intervention from 1992 to 1996 and then were followed with questionnaires and/or pathology departments and cancer registries through 2006. A borderline-statistically significant reduction in both total melanomas (HR 0.50, 95% CI 0.271.02; p = 0.051) and invasive melanomas (0.27, 95% CI 0.080.97; p = 0.045) in the intervention group. These findings support the long-assumed contention that sunscreen likely prevents the formation of invasive melanoma. In addition, a greater benefit might be expected if such a study such as this were performed in children, given that intense sun exposure and burns in childhood are a strong risk factor for developing melanoma (23). It is unlikely; however, that such a study would be performed given the ethical dilemma of randomizing children to not receive sunscreen intervention in areas of high incidence for melanoma.
PATHOLOGIC FEATURES OF MELANOMA
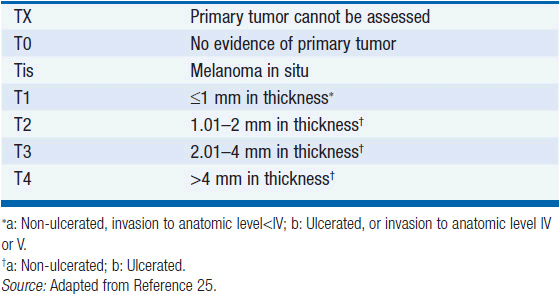
The likelihood of recurrence and death from melanoma is directly correlated with tumor thickness. Ulceration (the absence of an intact epidermal layer overlying the melanoma) is a powerful adverse prognostic feature. A high mitotic rate is also associated with a poor prognosis, and along with thickness and ulceration are the three factors incorporated into the American Joint Committee on Cancer staging criteria (24, 25).
Melanoma may arise de novo, from a preexisting nevus, or from melanoma in situ, in which the melanocytic proliferation is limited to the epidermis. Radial growth phase melanoma is confined in large part to the epidermis and has a low likelihood of dissemination. The vertical growth phase is characterized by prominent dermal invasion and signals the acquisition of metastatic potential.
Several distinct growth patterns of melanoma are recognized. Superficial spreading melanoma is defined by the presence of both a radial and a vertical growth phase, and accounts for up to 75% of melanomas. Nodular melanomas (15%–25%) are vertical growth phase lesions, located exclusively or predominantly in the dermis. Lentigo maligna melanoma typically arises from a noninvasive precursor lesion (lentigo maligna, or lentigo maligna melanoma in situ) and occurs most frequently on the face, scalp, or neck in older individuals. Acral lentiginous melanoma accounts for only 5% of melanomas but is the most common subtype in non-Caucasians. The sites of highest incidence are the palmar and plantar surfaces. Histologically, these lesions are characterized by the presence of nests of atypical melanocytes at the dermalepidermal junction, with infiltration of single cells or nests into the dermis.
A minority of melanomas are amelanotic, lacking obvious pigmentation, and mimic a variety of benign entities, often leading to a delay in diagnosis. In other respects their behavior is similar to pigmented melanomas.
The detection of melanocyte-associated antigens by immunohistochemistry may suggest or support the diagnosis of melanoma in difficult cases, such as metastatic cancer of uncertain histogenesis. Immunohistochemistry may also detect small deposits of metastatic melanoma within lymph nodes that are not evident on routine microscopic examination. S-100 is expressed by cells of melanocytic lineage, but also by histiocytes and certain neural tumors. Melan-A is also somewhat nonspecific. Antigens with a higher degree of specificity for melanocytes include tyrosinase, the microphthalmia transcription factor, and a protein in the premelanosome complex targeted by the monoclonal antibody HMB45. None of these antigens, however, can be used to distinguish melanoma from benign melanocytic proliferative processes.
STAGING AND PROGNOSTIC FACTORS
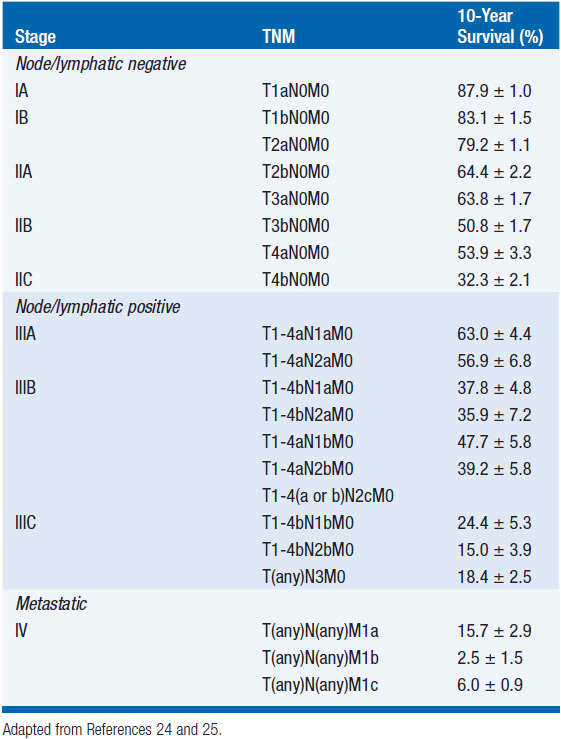
The American Joint Committee on Cancer’s 7th edition (2009) staging system for cutaneous melanoma is based on the analysis of prognostic factors in 30,946 patients (19). Stage I and II melanomas is defined as disease without regional lymphatic or systemic spread. The staging of node-negative melanoma is based on the worsening prognosis with increasing thickness of the primary lesion ulceration, and, for thin melanomas (≤1 mm), the presence of dermal mitoses (Table 60-1). While the majority of stage I and II melanomas are cured by surgery alone, even melanomas 1 mm or less in thickness, without ulceration or nodal involvement (T1aN0M0) have metastatic potential, and are associated with a 10-year disease-specific mortality rate of approximately 5%–10%. The 10-year survival in patients with thick melanomas (stages IIB and IIC) is 32.3%–53.9%. Ulceration is associated with a relative risk of death of 1.9 in node-negative melanomas.
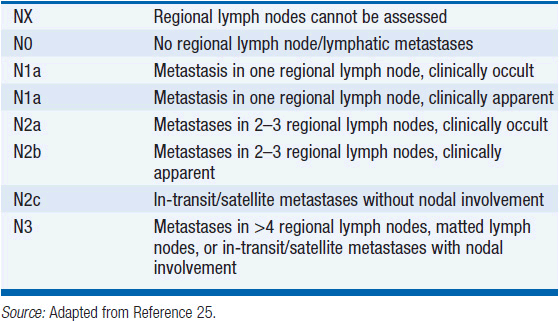
Stage III melanoma is defined by the presence of satellite and/or in-transit metastases, and/or involvement of regional lymph nodes (Table 60-2) in the absence of systemic metastasis. Lymphatic metastases within 2 cm of the primary lesion are designated satellite metastases; those located more than 2 cm from the primary melanoma, but before the first echelon of draining lymph nodes, are designated in-transit metastases. The burden of tumor in the lymph nodes is predictive of outcome and is represented by the number of nodes involved, and whether the involvement is microscopic (not clinically apparent prior to surgery) or macroscopic (clinically apparent).
While lymphatic involvement is associated with an increased risk of recurrence and death with each T stage subgroup, stage III melanoma is a heterogeneous disease. Specifically, patients with a thin (T1) or intermediate (T2), non-ulcerated melanomas with microscopic nodal involvement have a 10-year survival rate of approximately 70%, while patients with clinically detectable lymphadenopathy, <4 involved or matted lymph nodes, in-transit/satellite metastases, and/or ulceration of the primary melanoma and any degree of nodal involvement, have a 10-year survival that ranges from approximately 25% to 45%.
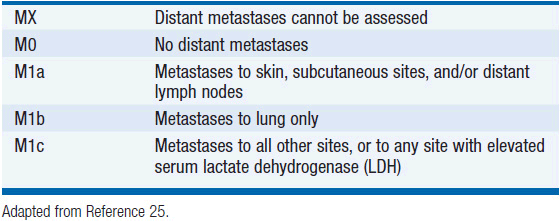
Stage IV melanoma represents distant metastatic spread, and is stratified according to sites of involvement and lactate dehydrogenase level (Table 60-3). Based on these T, N, and M criteria, patients can be grouped according to prognosis (Table 60-4).
Stage-for-stage, advanced age, and male sex are associated with a worse prognosis in melanoma (19). Melanomas of the extremities have a more favorable prognosis than those of the trunk. Head and neck melanomas, particularly those of the scalp and ears, have a worse prognosis than other sites (26).
SURGICAL MANAGEMENT
When a cutaneous lesion is suspected to be melanoma, biopsy techniques should be employed which preserve the pathologist’s ability to assess the thickness and depth of invasion of the lesion, that is, conservative excisional biopsy, or punch biopsy. Ablative procedures such as cryotherapy should be avoided.
Once the diagnosis of melanoma is established, a wide local excision is the treatment of choice. Five randomized trials have assessed the effect of margins of resection on local recurrence (27–31). For melanomas ≤1 mm in depth, local recurrence was not significantly different with 1 or 3 cm margins; therefore, 1-cm margins are acceptable for thin melanomas. For melanomas greater than 1 mm, the data generally support margins of 2 cm.
Randomized studies have failed to demonstrate a survival benefit for elective lymph node dissection in patients without clinically apparent nodal involvement, and this approach has largely been abandoned. The procedure of sentinel lymph node mapping and selective lymphadenectomy is highly sensitive for the detection of microscopic nodal metastases and has a high negative predictive value. Thus, patients without clinical evidence of nodal involvement (the majority) can be accurately staged without the morbidity of a complete nodal dissection, and patients harboring occult nodal disease can be identified. For patients with a positive sentinel node, a complete dissection of the involved lymph node basin(s) is usually recommended, although the benefit of this procedure has not been formally demonstrated. The detection of occult nodal metastases also identifies patients who may be candidates for adjuvant systemic therapy with interferon-alfa (high-dose or pegylated interferon) or participation in clinical trials.
In the Multicenter Selective Lymphadenectomy Trial, 1327 patients with melanomas 1.2–3.5 mm were randomized to wide excision with sentinel node mapping and biopsy, or wide excision followed by observation (32). Patients found to have positive sentinel nodes underwent completion node dissections. At a median follow-up of 59.8 months, the melanoma specific survival in the two groups was not significantly different (death from melanoma occurred in 13.8% of the observation group and 12.5% of the biopsy group). The benefit of sentinel node biopsy was reflected in risk of regional/nodal recurrence. Patients assigned to sentinel node biopsy had statistically superior disease-free survival compared with those who were observed (78.3% vs. 73.1%). Accounting for either microscopic nodal involvement (in the sentinel node biopsy cohort) or clinically evident nodal involvement (in the observation cohort), the overall rate of lymph node involvement was found to be very similar in both groups. Thus, sentinel node biopsy provided a basis for identifying those patients who warrant further lymph node surgery to prevent a clinically evident and potentially morbid regional recurrence.
ADJUVANT THERAPY FOR HIGH-RISK MELANOMA
The staging criteria described above identify patients at high risk for recurrence and death. Most studies of adjuvant therapies have focused on patients with intermediate thick, ulcerated primary melanomas (T3b), thick primary melanomas (<4 mm), and/or those with nodal involvement (stages IIB, IIC, and III), a group with an expected survival rate of less than 50%. Attempts to identify effective adjuvant therapy have been hampered historically by the absence of systemic therapies that produce meaningful response rates in advanced melanoma.
High-dose interferon alfa-2b (HDI) has been the subject of three phase III trials in high-risk, resected melanoma, yet controversy persists about its efficacy (33–35). A pooled, updated analysis of two of these trials showed a relapse-free survival advantage for HDI versus observation (HR = 1.30, p < 0.006) at a median follow-up of 7.2 years but no benefit for HDI in terms of overall survival (36). In a third trial, 880 patients with stage IIB–III melanoma were randomized to HDI or to vaccination with GM2, an immunogenic ganglioside expressed by melanoma cells. At a median follow-up of 2.4 years, there was a statistically significant advantage for HDI in terms of relapse-free and overall survival (35).
The impact of HDI on the risk of relapse seems confined to 20%–30% of patients, and its adverse effects are considerable. Recent investigations suggest a correlation between autoimmunity and a benefit from interferon.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree