Abstract
Ovarian stimulation is an integral part of many different infertility treatments. Since the 1970s, ovarian stimulation has been applied in ovulatory women diagnosed with unexplained infertility aiming to increase the number of developing follicles and the number of oocytes for fertilization in vivo. Ovarian stimulation is often combined with intrauterine insemination of sperm. Since the early 1980s, ovarian stimulation has become an essential part of in vitro fertilization (IVF) aiming to improve pregnancy rates by providing the laboratory multiple oocytes for fertilization and early embryo development. Medical ovulation induction has now matured providing good cumulative live birth rates. In skillful hands and with proper ovarian response monitoring, chances for complications are low for ovulation induction. The aim of this intervention is to mimic physiologic circumstances in anovulatory women, hence, single dominant follicle development and ovulation. However, a tendency to hyperrespond to ovarian stimulation is a well-known feature of polycystic ovary syndrome (PCOS). Moreover, recent studies have shown the usefulness of novel drugs such as the aromatase inhibitor, letrozole, next to the antiestrogen clomiphene citrate or exogenous gonadotropins. In everyday practice, ovulation induction is often ignored in favor of IVF, although no direct comparative trials have been reported to date.
Any form of ovarian stimulation increases the chances of pregnancy per cycle, but it is at the expense of increased complication rates, most importantly multiple pregnancies and ovarian hyperstimulation syndrome (OHSS). This holds especially true for ovarian stimulation aiming at maturing multiple dominant follicles for fertilization either in vivo. Various strategies may significantly reduce chances for OHSS.
Regarding IVF, numerous new treatment modalities have been introduced over the years―often with insufficient evidence of safety and efficacy―using different compounds and dose regimens for ovarian stimulation, gonadotropin-releasing hormone analogue cotreatment, oocyte maturation trigger, interventions preceding stimulation, and luteal phase supplementation. The most important clinical challenge is to find the right balance between improving chances for success (birth of a healthy child) with reasonable cost, acceptable patient discomfort, and a minimal complication rate. New developments are rendering ovarian stimulation less intense and more individualized.
Keywords
Ovulation induction, ovarian stimulation, polycystic ovary syndrome (PCOS), in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), intrauterine insemination (IUI), ovarian hyperstimulation syndrome (OHSS), follicle-stimulating hormone (FSH), luteinizing hormone (LH), androgens, antiestrogens, clomiphene citrate, aromatase inhibitors, letrozole, insulin sensitizers, metformin, gonadotropin-releasing hormone (GnRH) agonist, GnRH antagonist
Concepts of Ovarian Stimulation
- ◆
Ovarian stimulation can be applied for the medical treatment of anovulatory infertility (i.e., ovulation induction) or for infertility treatment in ovulatory women.
Ovarian stimulation is a central component of many infertility therapies. At the outset, it is important to emphasize that two different concepts of ovarian stimulation exist: ovulation induction and ovarian stimulation. These approaches differ in both the starting point (i.e., the type of patients treated) and end points (i.e., the aim of the medical intervention). The reader is referred to Chapters 22 and 31 for additional coverage of this topic.
Ovulation Induction
In the strict sense of the term, ovulation induction refers to the triggering of ovulation, that is, the rupture of the preovulatory graafian follicle and release of the oocyte. In the clinical context, however, this term refers to the type of ovarian stimulation for anovulatory women aimed at restoring normal fertility by generating normoovulatory cycles (i.e., to mimic physiology and induce single dominant follicle selection and ovulation). Ovulation induction represents one of the most common interventions for the treatment of infertility. Anovulation represents one of the few states of absolute infertility, but excellent cumulative pregnancy rates can be achieved if normal menstrual cyclicity is restored.
After the exclusion of intrinsic ovarian abnormalities (e.g., premature ovarian failure [POF] currently referred to as primary ovarian insufficiency [POI]), follicle development can be stimulated by various pharmacologic compounds, and normoovulatory cycles can usually be obtained. This can be achieved with appropriate monitoring of ovarian response and in the hands of skillful clinicians. Because of various more subtle inherent ovarian abnormalities in most of these women, especially in patients suffering from polycystic ovary syndrome (PCOS) along with the major individual differences in ovarian response to stimulation, the risks of multiple pregnancy and ovarian hyperstimulation syndrome (OHSS) are considerable. However, the occurrence of these complications can be reduced to an acceptable level. The therapeutic window for an acceptable ovarian response is small, with a major individual (and to some extent cycle to cycle) variability in response. Approaches for gonadotropin ovulation induction include slowly and prudently surpassing the individual follicle-stimulating hormone (FSH) threshold for ongoing follicle development, as will be discussed later in this chapter.
Many other approaches for ovulation induction are available. These approaches include interfering with negative estrogen feedback by using antiestrogens or aromatase inhibitors, the use of insulin-sensitizing agents, and laparoscopic surgical methods.
Ovarian Stimulation
This treatment modality has become an integral part of assisted reproductive technologies (ART). The aim of ART is to bring more male and female gametes closer together and thereby increase the chances of pregnancy. The goal of ovarian stimulation is to induce ongoing development of multiple dominant follicles and to mature many oocytes to improve chances for conception either in vivo (empirical ovarian stimulation with or without intrauterine insemination [IUI]) or in vitro with in vitro fertilization (IVF). This approach of interfering with physiologic mechanisms underlying single dominant follicle selection is usually applied in normoovulatory women. Although ovarian hyperstimulation can also be performed in anovulatory women, this approach should be clearly differentiated from ovulation induction. The physiologic concepts that underlie current approaches to ovulation induction and ovarian hyperstimulation are described later in this chapter.
Concepts of Follicle Development Relevant to Ovarian Stimulation
- ◆
Decreasing serum FSH concentrations during the follicular phase of the normal menstrual cycle are fundamental for single dominant follicle selection in the human.
Initiation of growth of primordial follicles, also referred to as primary recruitment, occurs continuously and in a random fashion and development from the primordial up to the preovulatory stage takes several months (see Chapter 8 ). The great majority of primordial follicles that enter this development phase undergo atresia prior to reaching the antral follicle stage. The regulation of early follicle development and atresia and the degree to which early stages of follicle development are influenced by FSH remain unclear, but evidence suggests that the transforming growth factor (TGF)-β superfamily and factors regulating apoptosis (i.e., programmed cell death) are involved. Only at more advanced stages of development do follicles become responsive to FSH and obtain the capacity to convert the theca cell–derived substrate androstenedione (AD) to estradiol (E 2 ) by the induction of the aromatase enzyme.
Owing to demise of the corpus luteum during the late luteal phase of the menstrual cycle, E 2 , inhibin A, and progesterone levels fall. This results in an increased frequency of pulsatile gonadotropin-releasing hormone (GnRH) secretion inducing rising FSH levels at the end of the luteal phase. Although each growing follicle may initially have an equal potential to reach full maturation, only those follicles that happen to be at a more advanced stage of maturation during this intercycle rise in FSH (levels surpassing the so-called threshold for ovarian stimulation) gain gonadotropin dependence and continue to grow ( Fig. 30.1 ). This process is referred to as cyclic, gonadotropin-dependent recruitment as opposed to the previously mentioned initial, gonadotropin-independent recruitment of primordial follicles.
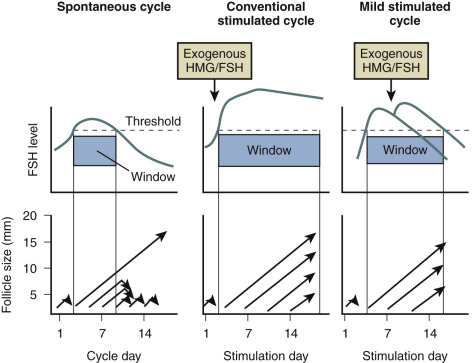
Based on indirect observations it is believed that the cohort size of healthy early antral follicles recruited during the luteofollicular transition is around 10 per ovary. During the subsequent follicular phase, FSH levels plateau during initial days and are gradually suppressed thereafter by ovarian inhibin B and E 2 negative feedback. A rise in inhibin B occurs just after the intercycle rise in FSH. It may therefore be proposed that inhibin B limits the duration of the FSH rise. Decremental follicular phase FSH levels (effectively restricting the time when FSH levels remain above the threshold, referred to as the FSH window ) (see Fig. 30.1 ) appear to be crucial for selection of a single dominant follicle from the recruited cohort. Only one follicle escapes from atresia by increased sensitivity for stimulation by FSH and luteinizing hormone (LH). This important concept of increased sensitivity of the dominant follicle for FSH has been confirmed by human studies showing developing follicles to exhibit a variable tolerance for GnRH antagonist-induced gonadotropin withdrawal. On the other hand, early stages of follicle development being independent from gonadotropins is confirmed in hypophysectomized women presenting with preovulatory graafian follicles within 2 weeks after the initiation of ovarian stimulation with exogenous gonadotropins.
A central role has also been demonstrated for LH in monofollicular selection and dominance in the normal ovulatory cycle. Although granulosa cells from early antral follicles respond only to FSH, those from mature follicles also contain LH receptors and therefore become responsive to both FSH and LH. The maturing dominant follicle may become less dependent on FSH because of the ability to respond to LH. It is suggested that the leading follicle continues its development owing to LH responsiveness, whereas smaller follicles enter atresia because of insufficient support by decreasing FSH concentrations during the late follicle phase. The dominant follicle can be distinguished by ultrasound from other cohort follicles by a size greater than 10 mm diameter. The concept of both endocrine and autocrine upregulation is supported by several other observations that characterize the dominant follicle, including the in vitro induction of aromatase enzyme activity, ovarian morphology, and endocrine changes in follicle fluid and serum. These observations all show that enhanced E 2 biosynthesis is closely linked to preovulatory follicle development.
These concepts of follicular development and selection have come to underlie contemporary approaches to therapeutic ovulation induction in women suffering from anovulatory infertility. Moreover, our increasing understanding of the processes underlying monofollicular selection has enabled the development of new approaches to ovarian hyperstimulation for assisted reproduction treatments.
Preparations Used for Ovarian Stimulation
- ◆
The history of ovarian stimulation with exogenous compounds in the human goes back almost a century. Many compounds and regimens have subsequently been developed.
- ◆
The history is of interest, and there are a few Nobel prizes in that historical recounting, but it does not guide current practice/patient care, so somewhat elective.
Evidence of the endocrine pituitary-gonadal axis arose early in the 20th century, when it was observed that lesions of the anterior pituitary resulted in atrophy of the genitals. The first convincing evidence supporting the existence of two separate gonadotropins (initially referred to as Prolan A and Prolan B) was provided by Fevold and Hisaw in 1931, and both LH and FSH were subsequently isolated and purified. In 1928, Aschheim and Zondek described the capacity of urine from pregnant women to stimulate gonadal function. The concept of stimulating ovarian function by the exogenous administration of gonadotropin preparations has intrigued investigators for many decades. As early as 1938, Davis and Koff had already described the ability of purified pregnant mare serum to induce ovulation in humans by intravenous administration. However, these initial attempts had to be stopped due to species differences resulting in antibody formation impacting efficacy and safety. Not until 1958 did Gemzell describe the first successful use for ovulation induction of gonadotropin preparations derived from human pituitaries. Shortly thereafter, Lunenfeld reported the clinical use of gonadotropin extracts from urine of postmenopausal women (for a historical overview, see Gruhn and Kazer and Lunenfeld ).
A second important development allowing for ovarian stimulation on a large scale was a fine example of medical serendipity. The first estrogen antagonist tested in cancer patients was found to induce ovulation.
Clomiphene Citrate
In the late 1950s, the first nonsteroidal estrogen antagonist (MER-25) was tested in patients to assess the efficacy of the compound in women with cystic mastitis, breast cancer, endometrial hyperplasia, or endometriosis. Some of these women with endometrial hyperplasia were of reproductive age and suffering from long-standing amenorrhea due to the Stein-Leventhal syndrome. To the great surprise of the investigators, the initiation of the medication in these women was followed by the recommencement of menstrual cycles. Shortly thereafter, the ovulation-inducing capacity of the next generation of closely related antiestrogens (MRL/41; clomiphene citrate [CC]) ( Fig. 30.2 ) was recognized. More than half a century later, CC is still the most applied drug for infertility therapies worldwide, accounting for around two-thirds of all prescriptions.
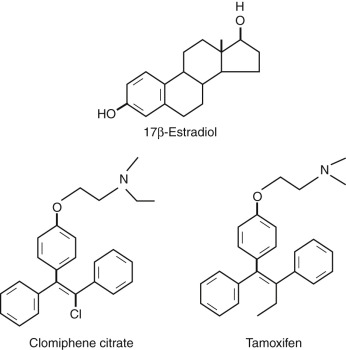
CC is a racemic mixture of two stereoisomers. The enclomiphene isomer has a relatively short half-life, whereas the zuclomiphene isomer has an extended clearance. The two isomers demonstrate different patterns of agonistic and antagonistic activity in vitro. Stimulation of ovarian function is elicited by raised pituitary FSH secretion due to blockage of E 2 steroid feedback by CC. Overall a 50% to 60% increase of serum FSH levels above baseline has been described. The exact nature of the mechanism of action of CC is still uncertain. Induced changes in other systems, such as insulin-like growth factor (IGF), may partly explain the capacity of CC to stimulate the ovary. However, antiestrogenic effects at the uterine level (cervical mucus production and endometrial receptivity) are believed to underlie the observed discrepancy between achieved ovulation and pregnancy rates. The impact of a concomitant rise in LH on ovarian response to CC is also uncertain. CC for ovulation induction is considered to be relatively safe because steroid negative feedback remains intact. The oral route of administration and low costs represent additional advantages of this preparation. CC was originally developed for clinical use by the Merrel company in 1956, and it is still considered to represent the first-line treatment strategy in most anovulatory infertility. In addition, this compound was a central component in the early days of IVF and is still often applied for the empirical treatment of unexplained infertility.
Gonadotropin Preparations
Clinical experiments in the late 1950s demonstrated that extracts derived from the human pituitary could be used to stimulate gonadal function. Subsequently, experiments involving the extraction of both the gonadotropic hormones LH and FSH from urine of postmenopausal women led to the development of human menopausal gonadotropin (hMG) preparations. From the early 1960s these preparations were used for the stimulation of gonadal function in the human. It soon became clear that hMG was a very potent compound. Its ability to directly stimulate the ovaries was accompanied by the inherent risks of ovarian hyperstimulation. Initial use in the treatment of anovulation was associated with high rates of multiple pregnancy and OHSS. The potential for dangerous complications induced the need for monitoring of ovarian response and dose adjustment. More recently introduced low-dose protocols applied in conjunction with intense ovarian response monitoring have substantially contributed to improved treatment outcomes.
Initial attempts by Edwards and Steptoe to enable the conception of a baby through IVF also involved hMG stimulation protocols. Because of a lack of pregnancies (presumed due to abnormal luteal function) it was decided to switch to natural cycle IVF. It was an unstimulated cycle that led to the conception of the first IVF baby, Louise Brown, who was born on July 25, 1978. Subsequent IVF pregnancies were reported from Australia to occur after ovarian stimulation with CC. The more widespread use of hMG for successful IVF was developed thereafter in the United States. For over 2 decades, gonadotropin preparations have also been extensively applied for ovarian stimulation in ovulatory women for empirical treatment of unexplained subfertility. The aim here is to increase monthly fecundity rates by increasing the number of oocytes available for fertilization in vivo (with or without the additional use of IUI). These trends and the rapid expansion in the use of IVF treatment underlie the enormous increase in worldwide demand and sales for gonadotropin preparations.
The early extraction techniques were very crude, requiring around 30 L of urine to manufacture enough hMG needed for a single treatment cycle. The FSH to LH bioactivity ratio of these early preparations was 1 : 1. These initial preparations were very impure with many contaminating proteins; only less than 5% of the proteins present were bioactive. As purity improved, it was necessary to add human chorionic gonadotropin (hCG) to maintain this ratio of bioactivity. Bioactivity of gonadotropin preparations continues to be assessed by the crude in vivo rat ovarian weight gain Steehlman and Pohley assay. This rather anachronistic technique has the disadvantage of allowing considerable batch-to-batch inconsistency in bioactivity.
Improved protein purification technology allowed for the production of hMG with reduced amounts of contaminating nonactive proteins and eventually the development of purified urinary FSH (uFSH) preparations by using monoclonal antibodies since the late 1980s. The currently available pure products allow for less hypersensitivity reactions, and less painful subcutaneous administration. Because of the worldwide increased need for gonadotropin preparations, demands for postmenopausal urine increased tremendously and adequate supplies could no longer be guaranteed. In addition, concern regarding the limited batch-to-batch consistency along with possibilities of urine contaminants emerged.
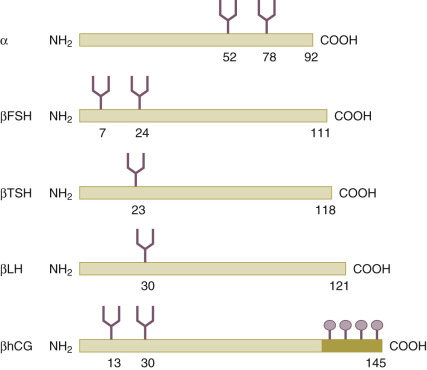
Through recombinant DNA technology and the transfection of human genes encoding for the common α subunit and hormone-specific β subunit of the glycoprotein hormone ( Fig. 30.3 ) into Chinese hamster ovary cell lines, the large-scale in vitro production of human recombinant FSH (recFSH) has been realized (see Chapter 2 ). The first pregnancies using this novel preparation in ovulation induction and in IVF were reported in 1992. Since then, numerous large-scale, multicenter studies have been undertaken, demonstrating their efficacy and safety. The recombinant products offer improved purity, consistency, and large-scale availability. Because of its purity, recFSH can now be administered by protein weight rather than bioactivity, and so-called filled-by-mass preparations are now available for clinical use. Subsequently, recombinant LH (recLH) and recombinant hCG (rechCG) have also been developed and introduced for clinical application. Finally, a long-acting recFSH agonist (a man-made chimeric hormone generated by the fusion of the carboxy-terminal peptide [CTP] of hCG to the FSH-β chain) has recently been introduced into the clinic after efficacy and safety had been established in large sample size trials where IVF clinics from all over the world participated. Moreover, the first recFSH produced by a human cell line has recently been tested, and recFSH biosimilars have been introduced on the market.
Gonadotropin-Releasing Hormone Analogues
In 1971, the small decapeptide GnRH was isolated and its structure was elucidated by Schally and Guillemin ( Fig. 30.4 ). Some years later, both investigators jointly received the Nobel Prize for this discovery. Amino acid substitutions have revealed the significance of specific regions for its stability, receptor binding, and activation of the gonadotrope cells. This decapeptide is secreted by the hypothalamus into the portal circulation in an intermittent fashion, stimulating the pituitary gonadotropes to synthesize and secrete LH and FSH. Early studies demonstrated that pituitary downregulation could be induced by the continued administration of GnRH.
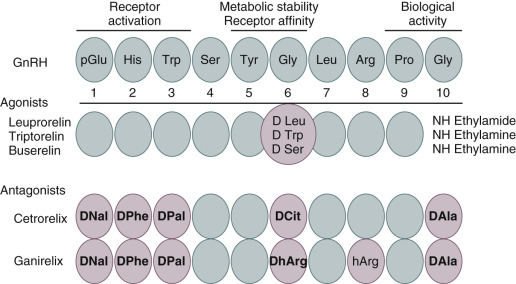
Clinically safe GnRH agonists were developed relatively easily by replacing one or two amino acids. An increased potency could be achieved by replacing glycine for D-amino acids at position 6 and by replacing Gly-NH 2 at position 10 by ethylamide. Such simple structural changes render these compounds more hydrophobic and more resistant to enzymatic degradation. The administration of GnRH agonists induces an initial stimulation of gonadotropin release for 2 to 3 weeks (the so-called flare effect ) followed by a downregulation (or desensitization) due to the clustering and internalization of pituitary GnRH receptors.
GnRH agonists have been used clinically since 1981 to induce a “chemical castration” for steroid-dependent disease states such as fibroids and endometriosis in females and prostate cancer in males. The first paper concerning its use in IVF for the prevention of a premature LH rise also appeared in the early 1980s. Shortly thereafter, the use of GnRH agonists such as buserelin, triptorelin, or leuprorelin to downregulate the pituitary prior to administration of gonadotropins (a strategy that became known as the “long protocol”) became the standard of care. The more recent clinical introduction of GnRH antagonists has slowly changed practice in IVF, and currently over 50% of IVF cycles apply GnRH antagonist cotreatment.
It has taken almost 3 decades to develop GnRH antagonists with acceptable safety and pharmacokinetic characteristics. The first-generation antagonists were developed by replacing amino acids histidine at position 2 and tryptophan at position 3, but these compounds suffered from low potency. In second-generation compounds, the activity was increased by incorporating a D-amino acid at position 6. However, the widespread clinical application of these compounds was hampered by frequent anaphylactic responses due to histamine release. By introducing further replacements at position 10, third-generation compounds were developed. Subsequently, both the compounds ganirelix and cetrotide were shown to be safe and efficacious in IVF. These third-generation GnRH antagonists were registered in 2001 for use in IVF. The immediate suppression and recovery of pituitary function renders these compounds appropriate for short-term use in IVF. Clinical uptake of GnRH antagonists in IVF has been rather slow. The most recent meta-analysis has confirmed the use of GnRH antagonist cotreatment to be effective and safer.
Outcomes of Ovarian Stimulation
- ◆
Ovarian stimulation is a common intervention in infertility.
- ◆
Ovarian stimulation may be applied in normoovulatory women for empirical reasons or in the context of IUI or IVF.
- ◆
Another form of ovarian stimulation involves the medical treatment of anovulatory infertility aiming to restore normal ovarian function.
Ovulation Induction
Amenorrheic women with anovulation exhibit virtually no chance of spontaneous conception, and ovulation induction may restore normal fertility. However, the aim of mimicking normoovulatory cycles cannot always be achieved, so the chances of complications such as multiple pregnancy or OHSS should be taken seriously, especially in patients diagnosed with PCOS. Oligomenorrheic women may or may not have incidental spontaneous ovulations; therefore spontaneous pregnancies may occur. For obvious reasons, fertility specialists see only oligo/amenorrheic women who have failed to conceive, and these patients will usually respond well to ovulation induction. The balance between success and complications resulting from ovulation induction is dependent on many factors, including patient characteristics, type of drugs used, gonadotropin preparations and dose regimens used, the intensity of monitoring ovarian response to stimulation, and willingness to cancel the cycle in case of hyperresponse. An alternative option under those circumstances would be to convert ovulation induction to IVF. Cumulative live birth rates of ovulation induction have been reported to be around 75% to 80%, with a coinciding incidence of multiple pregnancies of around 10% and of OHSS of less than 2%.
OHSS is a potentially life-threatening complication characterized by ovarian enlargement, high serum sex steroids, and extravascular fluid accumulation, primarily in the peritoneal cavity. In severe cases, hypotension, increased coagulability, reduced renal perfusion, and oliguria may occur. Deranged liver function tests, venous and arterial thrombosis, renal failure, and adult respiratory distress syndrome can ensue, and fatalities have been reported. Moderate to critical OHSS is very rare with CC but constitutes an important complication of gonadotropin use. The incidences of mild, moderate, and severe OHSS following gonadotropin ovulation induction have been reported to be 20%, 6% to 7%, and 1% to 2%, respectively. In addition to PCOS, risk factors for the development of OHSS include young age and low body weight. The risk is further increased when adjuvant GnRH agonist treatment is employed.
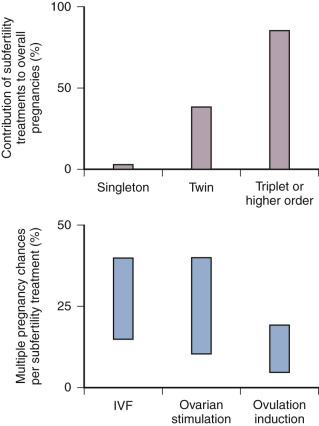
The contribution of ovulation induction treatment to the number of triplet and higher-order pregnancies is considerable. It has been calculated that 40% of higher-order multiple births in the United States could be attributed to the use of ovulation-inducing drugs without assisted reproduction.
Ovarian Stimulation
As previously outlined, the aim of ovarian stimulation alone or in combination with assisted reproductive techniques is to bring an increased number of gametes (oocytes and sperm) in close proximity to augment pregnancy chances. Ovarian stimulation alone may give rise to a two- to fourfold increase in pregnancy rates. The associated risk of OHSS and the occurrence of twin and higher-order multiple births are dependent on the magnitude of ovarian stimulation, the intensity of ovarian response monitoring, and the criteria applied for cycle cancellation should too many follicles develop. The overall incidence of severe ovarian OHSS associated with ovarian hyperstimulation is less than 5%.
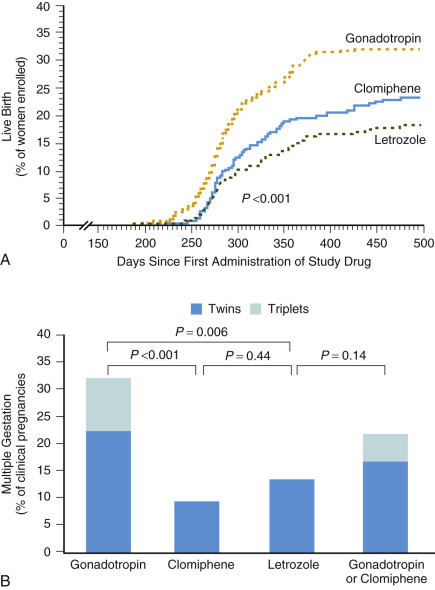
Initial studies suggested that a threefold increase in monthly probability of pregnancy can be achieved with empirical ovarian stimulation in the treatment of unexplained infertility ( Fig. 30.5 ). Subsequently a large multicenter study showed that ovarian hyperstimulation with gonadotropins and IUI both exhibit an independent additive effect on pregnancy chances. Moreover, overall cumulative pregnancy rates with this combined therapy were reported to be 33% within three cycles, but at the price of an unacceptably high multiple pregnancy rate of 20% for twins and 10% for higher-order multiple pregnancy. It has been proposed that a similar cumulative pregnancy rate could be achieved by expectant management over a 6-month period, obviously with much lower chances of multiple pregnancy. A recent, well-designed randomized controlled trial (RCT) comparing ovarian stimulation in unexplained infertility comparing gonadotropins, the aromatase inhibitor letrozole, and CC concluded gonadotropins to be superior in terms of cumulative clinical pregnancy rates, but again at the expense of a high multiple pregnancy rate of 32% ( Fig. 30.6 ). Less intense ovarian stimulation may reduce the incidence of higher-order multiple pregnancies, but probably at the expense of a reduction in overall conception rate. Based on a 2-year experience in a large US infertility clinic involving 3347 consecutive ovarian stimulation cycles (ovulation induction and ovarian hyperstimulation combined) in approximately 1500 women, a 30% pregnancy rate was described. Twenty percent of these pregnancies were twins, along with 5% triplets and 5% quadruplets or higher order. The most worrying conclusion of this analysis was that the number of large antral follicles or serum E 2 levels during the late follicular phase had only limited value in predicting higher-order multiple gestations. The true rate of multiple pregnancies arising from ovarian stimulation with or without IUI remains uncertain, however, as few national registers record the outcome of ovarian stimulation. A recent summary of the European IVF monitoring consortium involving 34 countries, and a total of over 640,000 treatment cycles for the year 2012, reported a multiple delivery rate of 17.9%.
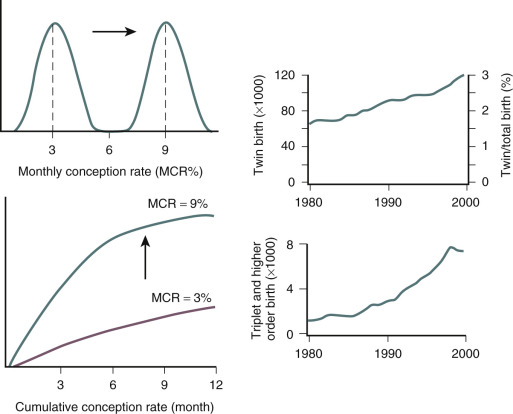
A number of years ago, it was estimated that ovarian stimulation with or without IUI is responsible for around 30% of multiple births ( Fig. 30.7 ). It is easier to influence chances for multiple gestations after IVF, because the occurrence is primarily dependent on the number of embryos transferred. Therefore ovarian stimulation for IVF is merely the factor allowing for the generation of multiples, but it is not the sole determining factor as in IUI. Unsurprisingly, the incidence of twin pregnancies following IVF without stimulation or with ovarian stimulation combined with single embryo transfer (SET) is close to normal. Over the years, the number of embryos transferred in IVF has decreased globally, but two to three embryos are still transferred in a significant proportion of IVF cycles in many countries around the world.
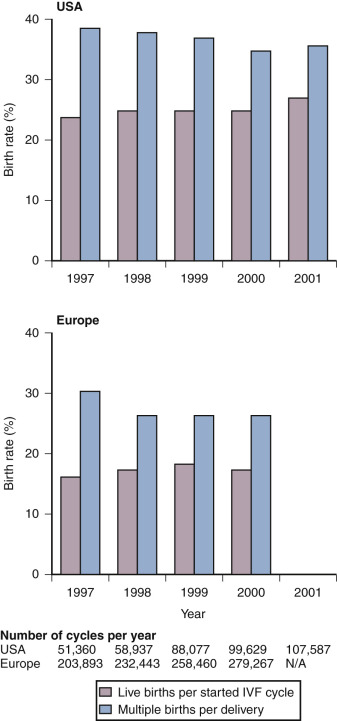
On the basis of a large nationwide data set from the United Kingdom, it was reported that the number of embryos transferred could be reduced from three to two without a concomitant drop in overall pregnancy chances. The policy of two embryo transfer was adopted by many major European IVF centers during the 1990s. Subsequently it was demonstrated that, in young women in whom two high-quality embryos are transferred, the chances of a twin pregnancy are actually higher than for a singleton pregnancy. The great majority of centers in Northern Europe have now adopted a SET policy in the great majority of IVF patients. Not surprisingly the multiple pregnancy rate dropped dramatically, but surprisingly the cumulative (fresh and frozen embryo transfer from the same oocyte harvest) live birth rate remained the same. In general, overall IVF results in Europe remain slightly lower compared to the United States but with an overall reduced incidence of multiple and premature birth ( Fig. 30.8 ).
Given the risks associated with ovarian stimulation, couples should be well counseled regarding their spontaneous chances for pregnancy prior to commencing empirical therapy for unexplained infertility ( Table 30.1 ). These chances are often underestimated.
| Category | MFR (%) | Cumulative Pregnancy Rate After (%) | |||
|---|---|---|---|---|---|
| 6 Months | 12 Months | 24 Months | 60 Months | ||
| Superfertile | 60 | 100 | — | — | — |
| Normally fertile | 20 | 74 | 93 | 100 | — |
| Moderately subfertile | 5 | 26 | 46 | 71 | 95 |
| Severely subfertile | 1 | 6 | 11 | 21 | 45 |
| Infertile | 0 | 0 | 0 | 0 | 0 |
Higher-order multiple pregnancies have a major adverse impact on perinatal morbidity and mortality rates. Mortality rate is increased 4- to 7-fold in twins and up to 20-fold in triplets. Children born from multiple pregnancies have more chances for perinatal complications and subsequent health problems, chiefly associated with prematurity and low birth weight. Chances for cerebral palsy are increased almost 50-fold in children from triplet pregnancies. Even the second child from a twin pregnancy delivered at term presents with a significant increased risk for death due to complications of vaginal delivery. Besides the medical and emotional burden, the financial costs associated with multiple pregnancies should be considered by policy makers. Obstetric and neonatal costs are increased fivefold to sevenfold in higher-order multiples, and by the age of 8, costs for low-birth-weight children are increased eightfold. Finally, possibilities of more subtle health risks that may be revealed only later in life should also be taken seriously.
Perhaps one strategy that may help improve the situation would be to agree to a new way of defining success from infertility therapy. The appropriate outcome measure may be shifted from pregnancy rate per treatment cycle toward health live birth started course of treatment (which may involve multiple treatment cycles) in the context of cost, burden of treatment, and complication rates.
Induction of Ovulation in Anovulatory Women
- ◆
The term ovulation induction should be reserved for the medical treatment of anovulatory infertility.
- ◆
Good results in terms of cumulative singleton live birth can be achieved by skilled hands and with proper ovarian response monitoring.
- ◆
Trials directly comparing outcomes of ovulation induction versus IVF are urgently needed.
Principles of Ovulation Induction
The aim of induction of ovulation in anovulatory women is to stimulate a single follicle to develop up to the preovulatory stage and subsequently ovulate. As stated before, this therapeutic goal should be clearly distinguished from two other forms of ovarian stimulation. First, ovulatory women with unexplained infertility may undergo ovarian stimulation aimed at producing two or three follicles and an increased chance of fertilization in a given cycle. This treatment, which is frequently combined with IUI, is discussed later in the chapter. Second, ovarian stimulation may be applied in ovulatory women undergoing IVF treatment where multifollicular development is required to produce multiple oocytes.
In contrast, ovulation induction aims to mimic the normal physiologic monofollicular ovulatory cycle. Ovulation induction is characterized therefore by tighter therapeutic margins and a need for careful monitoring and skilled management if success without complications is to be achieved. Ovarian surgical techniques such as laparoscopic drilling offer an alternative to medical therapies in this context. Again, the aim of this treatment paradigm is to institute monofollicular ovulatory cycles.
Anovulatory disorders account for around 25% of causes of infertility. This proportion may increase with the rising prevalence of obesity. Anovulation is usually manifested as the absence (amenorrhea) or infrequent occurrence (oligomenorrhoea) of menstrual periods. Although oligomenorrhoea may be associated with occasional ovulation, the chance of a woman conceiving within a year of unprotected intercourse is clearly diminished unless therapeutic steps are taken. Many medical approaches have been developed to achieve the goal of inducing the monthly development of a single dominant follicle and subsequent ovulation. In recent years, increased understanding of the pathophysiology of ovarian dysfunction has enabled the development of clinical strategies that aim to mimic the endocrine control of normoovulatory cycles. Achieving this within the narrow therapeutic margins of stimulating single rather than multiple follicular developments remains a challenge to clinicians.
The second European Society of Human Reproduction and Embryology (ESHRE) and Association of Reproductive Managers (ASRM) sponsored PCOS consensus workshop acknowledged that much attention should be paid to the condition of the woman (in terms of food intake, lifestyle, and smoking habits) before embarking on any form of ovulation induction. Such a periconception strategy emphasizes the general observation that chances for pregnancy complications and compromised children outcomes are directly related to the health status of the woman before getting pregnant ( Boxes 30.1 and 30.2 ).
- •
Clomiphene versus letrozole as first-line treatment
- •
Should adjunct drugs be tried next to clomiphene citrate before second-line treatment?
- •
Efficacy and safety of gonadotropins for ovulation induction in everyday practice
- •
Short- and long-term pros and cons of ovarian surgery compared to gonadotropins ovulation induction
- •
Ovulation induction outcomes compared to in vitro fertilization as first-line treatment in terms of success, cost, burden of treatment, and complications
- •
Does generating more oocytes improve overall outcomes of IVF?
- •
Most appropriate starting does for FSH
- •
Is there enough evidence to individualize the FSH starting dose?
- •
Usefulness of changing the FSH dose during stimulation
- •
Usefulness of additional compounds (such as clomiphene, letrozole, androgens, LH/hCG, growth hormone, and others)
- •
Intensity and way of ovarian response monitoring
- •
Cotreatment with GnRH agonist/antagonist
- •
Pretreatment with steroids
- •
Is ovarian stimulation associated with impaired endometrial receptivity?
- •
Most appropriate intervention in case of low ovarian response
- •
Mild ovarian stimulation
- •
Oocyte maturation triggering by GnRH agonist trigger
- •
Luteal phase supplementation
FSH, Follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin; IVF, in vitro fertilization; LH, luteinizing hormone.
Classification of Anovulation
Ovarian dysfunction can be readily classified in everyday clinical practice based on the assessment of serum gonadotropin and estrogen levels in peripheral blood. This concise approach, known as the World Health Organization (WHO) classification of anovulation, was originally developed by Insler and colleagues. Amenorrhea may coincide with either low or normal E 2 , whereas oligomenorrhea is associated only with normal estrogens. Low estrogens combined with low gonadotropin levels suggest a central origin of the disease at the hypothalamic-pituitary level. This cause of anovulation occurs in less than 10% of infertile women and is termed WHO class 1. Low estrogens in combination with high gonadotropins suggest defective ovarian function per se, usually based on POF (currently referred to as POI) or ovarian dysgenesis. This cause of anovulation, termed WHO class 3, occurs in around 5% of infertile women and around 1% to 2% of the general female population. Anti-müllerian hormone (AMH) may help to identify women with POI with some residual ovarian activity.
The majority (80% to 90%) of anovulatory women present with estrogen and FSH levels within normal limits. LH levels may be increased in these women. PCOS exhibiting FSH and E 2 concentrations within the normal range represents the great majority of these women. Recently new criteria for the diagnosis of PCOS have been supported by the ASRM and ESHRE. The so-called Rotterdam consensus criteria are broader than the National Institutes of Health (NIH) criteria primarily because polycystic ovaries are now included. The incidence of PCOS as defined by the Rotterdam criteria is therefore higher.
An additional cause of anovulation with an endocrine etiology is hyperprolactinemia, which may present with normal or reduced gonadotropin and E 2 concentrations. This may be considered as a variant of WHO class 1 anovulation because high serum prolactin levels suppress GnRH release by the hypothalamus by altering opioid receptor stimulation. Hyperprolactinemia may also present with normal gonadotropin and E 2 concentrations and may then be considered as a variant of WHO class 2. The pathophysiology and treatment of hyperprolactinemia are discussed in detail in Chapter 3 .
Preparations for Treating Anovulation
- ◆
The medical treatment of anovulation can be performed using different drugs.
- ◆
The preferred drug should be viewed in the context of ease of use, cost, efficacy, and risks.
Antiestrogens
Background
The most widely used antiestrogen for treating anovulation is CC, the development and pharmacology of which are addressed in the introduction to this chapter. In terms of its relative efficacy, safety, cost, and ease of use, it remains some 50 years after its introduction into clinical practice the most important therapeutic agent in use. The principal indication for CC is the treatment of anovulatory infertility in women with an intact hypophyseal-pituitary-ovarian axis. In this role it remains the first-line therapy for most clinicians. Given orally in the early to midfollicular phase, it causes a 50% rise in the endogenous serum FSH level, thus stimulating follicle growth. This rise in FSH is accompanied by a similar rise in serum LH levels. Limitation of the duration of administration to 5 days is aimed at allowing FSH levels to fall in the late follicular phase and the mechanisms for monofollicular development and ovulation to operate. However, elevated gonadotropin levels may persist into the late follicular phase in some women. The long half-life zuclomiphene isomer (which exhibits predominant estrogen agonist activity) has been shown to persist and accumulate across consecutive cycles of treatment. However, the resulting concentrations are well below those demonstrated to have any effects in vitro and are unlikely to be of clinical significance.
Preparations and Regimens
The conventional starting dose of CC is 50 mg/day, starting from day 2 until day 5 of the menstrual cycle, for 5 consecutive days. In normogonadotropic amenorrheic women, treatment can be initiated following a progesterone-induced withdrawal bleeding. Whether CC is commenced on cycle day 1 or 5 does not appear to affect outcomes. Should 50 mg/day fail to elicit follicle growth, the dose should be increased to 100 mg/day in the subsequent cycle, followed by 150 mg/day, which is usually considered to be the maximum dose beyond which alternative treatments are indicated. The LH surge occurs between 5 and 12 days following the last day of CC administration. Intercourse is therefore advised for a week from the fifth day after the last day of CC administration. Some advocate hCG administration as a surrogate for the LH surge to trigger ovulation and to time intercourse. However, recent studies showed no improvement in outcomes, despite the increased monitoring required to time hCG administration.
Clinical Outcome
Between 60% and 85% of anovulatory women will become ovulatory with CC, and 30% to 40% will become pregnant. In a meta-analysis based on four placebo-controlled studies in oligomenorrheic patients, the odds ratio with CC was 6.8 for ovulation and 4.2 for pregnancy. Why some women with WHO class 2 anovulation do not respond to CC is not fully understood. Altered individual requirements for FSH at the ovarian level, the local intraovarian effect of autocrine or paracrine factors, and variations in FSH receptor expression or FSH receptor polymorphisms may contribute (see Chapter 2 ). A number of studies have pointed to being overweight as a negative factor. In a multivariate analysis of factors found to predict outcome of CC ovulation indication, the free androgen index (FAI), body mass index (BMI), presence of amenorrhea (as opposed to oligomenorrhea), and ovarian volume were found to be independent predictors of ovulation.
The occurrence of ovulation can be identified using temperature charts and midluteal urinary pregnanediol or serum progesterone measurements. Although results of large trials indicate that monitoring by ultrasound is not mandatory to ensure good outcomes, the practice in many centers is to monitor the first cycle to allow adjustment of dose where necessary. The cumulative pregnancy rate in ovulatory women with CC in 6 to 12 months of treatment is around 70%, with conception rates per cycle around 22%. Why do some women who become ovulatory with CC not conceive? Reasons include patient selection, the regimen used, and the presence of other causes of subfertility. The antiestrogenic effects of CC on the reproductive tract have been particularly implicated. Negative effects on tubal transport, quantity and quality of cervical mucus, and the endometrium have all been reported.
Miscarriage rates of 13% to 25% are reported. Although these numbers appear high, they are similar to the spontaneous miscarriage rate and those observed in infertile women undergoing IVF. In general, it does not appear that the miscarriage rate is significantly increased in anovulatory women treated with CC.
Side Effects and Complications
Apart from hot flushes, which may occur in up to 10% of women taking CC, side effects are rare. Nausea, vomiting, mild skin reactions, breast tenderness, dizziness, and reversible hair loss have been reported, but less than 2% of women are affected. The mydriatic action of CC may cause reversible blurred vision in a similar number. The multiple pregnancy rate is less than 10%, and OHSS is rare. The putative increased risk of ovarian cancer reported to be associated with the use of CC for more than 12 months has led CC to be licensed for just 6 months of use in some countries.
Similar to CC, tamoxifen is a nonsteroidal selective estrogen receptor modulator (SERM). In contrast to CC, tamoxifen contains only the zu-isomer and appears to be less antiestrogenic at the uterine level. The possible advantages of tamoxifen over CC include an agonistic effect at the endometrium. Many uncontrolled studies in the area of ovulation induction have suggested that tamoxifen may be a safe and efficacious alternative to CC. A meta-analysis of four randomized controlled studies revealed tamoxifen to be as effective as CC in inducing ovulation. However, despite the theoretical benefits, no significant improvement in pregnancy rates was observed compared with CC. Clinicians should therefore base their choice of treatment on familiarity with the given regimen.
Insulin-Sensitizing Agents
Background
The role of insulin resistance in the pathogenesis of ovarian dysfunction in many PCOS patients has led to the introduction of insulin-sensitizing agents as adjuvant or sole treatment regimens for the induction of ovulation. The most extensively studied insulin-sensitizing drug in the treatment of anovulation is metformin. Metformin (dimethylbiguanide) is an orally administered drug used to lower blood glucose concentrations in patients with non–insulin-dependent diabetes mellitus (NIDDM). It is antihyperglycemic in action and increases sensitivity to insulin by inhibiting hepatic glucose production and by increasing glucose uptake and utilization in muscle. These actions result in reduced insulin resistance, lower insulin secretion, and reduced serum insulin levels.
Many papers have been published initially advocating the clinical usefulness of this compound for ovulation induction. The absence of well-designed and properly powered studies did not dampen enthusiasm for metformin in this context, and it has been widely introduced into clinical practice. Recently, however, two large, placebo-controlled randomized studies comparing metformin to CC and metformin as adjunctive therapy to CC have shown no benefit of metformin ( Fig. 30.9 ).

Preparations and Regimens
The first studies reporting the use of metformin as an ovulation induction agent suggested that metformin improved insulin sensitivity, lowered LH and total and free testosterone concentrations, and increased FSH and sex hormone–binding globulin levels. This and subsequent uncontrolled studies indicated that correction of hyperinsulinemia has a beneficial effect in anovulatory women, by increasing menstrual cyclicity, improving spontaneous ovulation, and thus promoting fertility. It is recommended that metformin be commenced at 500 mg/day orally, rising to 500 mg 3 times a day over 7 to 10 days. Depending on response, this may be increased to 1000 mg twice a day. The optimal duration of treatment remains unclear. However, most studies reporting a beneficial effect from metformin have shown this within 2 to 4 months.
Clinical Outcome
The majority of studies on the outcome following metformin therapy are small and uncontrolled or simply case series. Most of the available data on restoration of menses following metformin therapy are on predominantly obese hyperinsulinemic women with PCOS. Similarly, studies of the ability of metformin to induce ovulation have been primarily carried out in obese women. In a meta-analysis of 15 studies involving 543 participants with PCOS, metformin was found to be effective in achieving ovulation with odds ratios of 3.88 (CI, 2.25 to 6.69) for metformin versus placebo and 4.41 (CI, 2.37 to 8.22) for metformin and clomiphene versus clomiphene alone. However, a recent large multicenter study has clarified the role of metformin as an alternative first-line ovulation induction agent in women with PCOS. In this study, 626 women with PCOS were randomized to receive 50 to 150 mg CC plus placebo from cycle day 3 to 7500 to 2000 mg daily doses of extended release metformin plus placebo or a combination of metformin and CC. Treatment was continued for up to 6 months. Obesity was not an exclusion criterion. The results of this study are summarized in Fig. 30.9 . The primary end point of live birth rate was 22.5% after treatment with CC compared with a significantly lower rate of just 7.2% following metformin treatment. Combination therapy with both metformin and CC yielded a live birth rate of 26.8%, which did not differ significantly from that achieved with CC treatment alone. A significant proportion of women using metformin discontinue treatment because of side effects. Pharmacogenomic studies on genes involved in metformin transport and action and effectiveness of in ovulation induction have been reported, but the findings are not yet translatable to clinical application.
It has been suggested that metformin may reduce the rate of miscarriage compared with CC-derived pregnancies. However, in the study of Legro and colleagues, the rate of first trimester loss did not differ significantly between the treatment groups, although the study was not powered to detect this.
Metformin therapy has also been proposed to aid weight loss in obese women with PCOS. Many studies have now examined the effect of metformin on BMI, and the evidence is conflicting. However, the majority of observational studies addressing weight loss with metformin have revealed a reduction in the BMI of 1% to 4.3%. More recently, a double-blinded randomized trial compared metformin 850 mg twice daily treatment with placebo in 143 PCOS women with a BMI greater than 30. After 6 months’ treatment, no significant difference in weight loss or menstrual frequency was observed. In contrast, lifestyle modification was to improve cycle regularity by improving weight loss.
Attention has turned in recent years to the possible benefits and safety of metformin administration during pregnancy. PCOS pregnancies demonstrate a greater incidence of perinatal and maternal complications such as gestational diabetes, preeclampsia, and premature delivery ( Box 30.3 ). A number of studies have appeared suggesting a role for metformin to ameliorate these complications. However, conflicting results have been reported and most of these studies are not randomized or suffer from small numbers and surrogate outcomes.
Aromatase Inhibitors
Background
In recent years the use of aromatase inhibitors to mimic the actions of CC has been proposed. Rather than antagonizing estrogen feedback activity at the hypothalamic-pituitary axis, this approach aims at reducing the amount of estrogens being synthesized. Aromatase inhibitors block the conversion of AD and T to estriol (E 3 ) and E 2 , respectively. This increases gonadotropin secretion, resulting in stimulation of ovarian follicles. Aromatase inhibitors have been in clinical use for more than 20 years, primarily in the treatment of postmenopausal patients with advanced breast cancer. The more recently developed third generation of aromatase inhibitors are characterized by their potency in inhibiting the aromatase enzyme without significantly inhibiting inhibition of other steroidogenesis enzymes. One of the third-generation compounds, letrozole, has been the focus of study as a potential therapeutic agent for inducing ovulation.
Clinical Outcomes
When given in the early follicular phase, letrozole reduces estrogen feedback at the pituitary-hypothalamic axis, causing a subsequent increase in gonadotropin secretion. This was shown in monkeys to stimulate follicle development. Subsequent small clinical studies employing a dose of 2.5 mg/day from day 3 to day 7 of the menstrual cycle have suggested that it may be an effective ovulatory agent in CC-resistant women. A local effect at the ovary to increase sensitivity to FSH by blocking the conversions of androgens to estrogens has also been proposed, because accumulating intraovarian androgens may increase FSH receptor gene expression.
Although the concept of applying aromatase inhibitors as an alternative to CC or as adjuvant therapy to CC or gonadotropins seems attractive and preliminary data on pregnancy outcome were encouraging, a more recent systematic review and meta-analysis, involving 13 RCTs, concluded that aromatase inhibitors should not be recommended as first-line ovulation induction treatment in the absence of good quality evidence of efficacy.
A large sample size RCT published in 2014, performed by the NICHD Reproductive Medicine network, comparing letrozole and CC as first-line treatment in 750 women diagnosed with PCOS, surprisingly reported a cumulative live birth rate of 28 versus 19%, respectively ( Fig. 30.10 ). Altogether, based on current knowledge, letrozole can be considered a realistic alternative for first line ovulation induction in PCOS. However, the generalizability in these findings remains a matter of debate since the study was performed in a severely obese population, and resulting CC success rates are much lower than reported elsewhere.
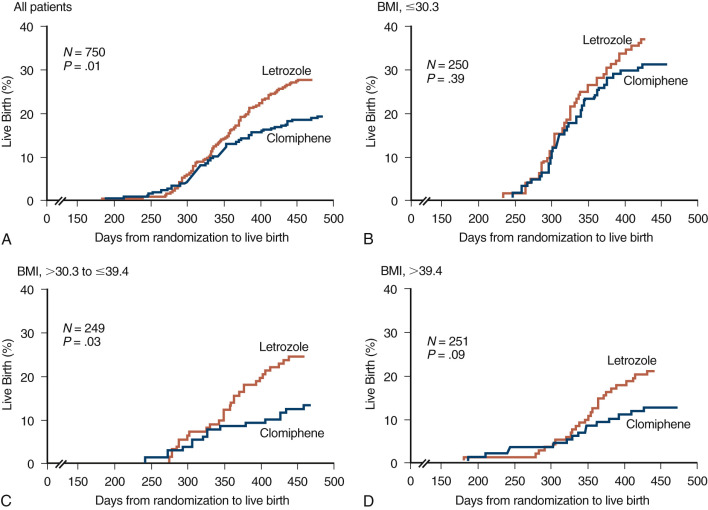
Adverse Effects and Complications
Although letrozole has a half-life that allows rapid disappearance following cessation of treatment in the midfollicular phase, the possible effects of this drug on ensuing pregnancy remain to be clarified. The enthusiasm for undertaking additional clinical studies has been inhibited because an association was reported between letrozole and fetal toxicity. However, a more recent analysis of outcomes in 911 newborns conceived following CC or letrozole treatment showed no difference in the overall rates of major and minor congenital malformations.
Gonadotropins
Background
Women with WHO class 2 anovulation who fail to ovulate or conceive following ovulation induction with antiestrogens can be successfully treated with exogenous gonadotropins. Exogenous gonadotropins have been widely used for the treatment of anovulatory infertile women since 1958. Improvements in purification techniques led to increasing relative amounts of the active ingredients, and the first urine-derived preparation containing only FSH (uFSH) became available in 1983. The development and application of production techniques based on immunoaffinity chromatography with monoclonal antibodies enabled the production of highly purified uFSH. In the 1980s, recombinant DNA technology led to the development and, later, the clinical introduction of human recFSH. This advance promised not only unlimited availability, but improved purity and batch-to-batch consistency compared to urinary derived products.
The development of recombinant gonadotropins also provided the opportunity to elucidate more clearly the physiology of ovarian E 2 synthesis. During further follicular development, LH has a synergistic action with FSH. Theca cells are stimulated by LH to convert cholesterol into AD and testosterone (T) by cytochrome P450 side chain cleavage oxidases and 3β-hydroxysteroid dehydrogenase. Aromatase activity in the granulosa cells is induced by FSH and converts AD and T into estrone and E 2 . The involvement of two cell types (granulosa and theca cells) and two hormones (LH and FSH) to produce estrogens from cholesterol has led to the “two-cell, two-gonadotropin” concept. In addition to stimulating aromatase activity, FSH also induces LH receptors and further increases FSH receptor formation on granulosa cells while stimulating DNA and protein synthesis by the cell. Clinical observations in the treatment of anovulatory women have supported this concept.
In the treatment of WHO class 1 (hypogonadotropic hypogonadal) anovulation, women with intact pituitary function can be treated with pulsatile GnRH therapy to restore the periodic release of FSH and LH. The treatment of hypogonadotropic women with FSH alone leads to follicular development but not pregnancy. Exogenous LH is therefore required to treat this form of anovulatory infertility. Until recently, hMG was the only source of exogenous LH for this group of patients. Now recLH or rechCG offers the possibility for a more sophisticated approach to treatment.
Recent studies have demonstrated the safety and appropriate dose required to affect follicle development and subsequent pregnancy. It has been established that baseline levels of at least 0.5 to 1 IU LH should be sufficient to provide maximal stimulation to thecal cells. In a study of hypogonadotropic women undergoing treatment with recFSH and recLH, a dose of 75 IU per day of recLH was observed to result in follicular development and pregnancy. However, further increases in LH levels above the threshold level needed to gain a response did not appear to induce a greater degree of ovarian stimulation.
Preparations and Regimens
In addition to urinary derived FSH products, recFSH has been clinically available since 1996 in the form of follitropin alpha and follitropin beta. More recently, a long-acting recFSH (corifollitropin alpha), a recLH, and a rechCG have been added to the clinical arsenal for ovarian stimulation.
To achieve development of a single dominant follicle with exogenous gonadotropins, specific treatment and monitoring protocols are needed. The most frequently encountered dose regimen in the literature and in clinical practice is the low-dose step-up protocol. The initially described standard step-up protocol used a starting dose of FSH 150 IU/day. However, this regimen was associated with a high complication rate. Multiple pregnancy rates of up to 36% were reported, and OHSS occurred in up to 14% of treatment cycles. As a result, this protocol has been abandoned.
The concept of the FSH threshold proposed by Brown postulated that FSH concentrations must exceed a certain level before follicular development will proceed (see Fig. 30.1 ). Once this level is reached, normal follicular growth requires only a minor further increase above this threshold. Exposure to excessive FSH serum concentrations may lead to excessive follicular development. This concept forms the theoretical basis for low-dose step-up regimens for ovulation induction. A low-dose, step-up protocol designed to allow the FSH threshold to be reached gradually has now become the most widely used regimen, reducing the risk of excessive stimulation and development of multiple preovulatory follicles. The recommended initial starting dose of FSH is 37.5 to 50 IU/day. The dose is increased by 50% if no response is observed on ultrasonography after 14 days (and serum E 2 monitoring). The detection of an ovarian response is an indication to continue the current dose until hCG can be given to trigger ovulation. If equal daily doses of FSH are given from the beginning of the follicular phase, steady-state serum FSH concentrations are reached after 5 to 7 days. During step-up regimens, elevated FSH serum concentrations may occur during the late follicular phase, which may, in a similar manner, interfere with selection of a single dominant follicle. Previous suppositions that steroid negative feedback remained intact during low-dose step-up regimens have not been substantiated by scientific data.
In contrast to the concept of the FSH threshold on which the low-dose step-up protocol is based, the concept of the FSH “window” stresses the significance of the duration of FSH elevation above the threshold level rather than the magnitude of elevation of FSH for single dominant selection. This concept was substantiated by the demonstration that elevating FSH levels high above the threshold level for a short period of time in the early follicular phase does not increase the number of dominant follicles. Conversely, when the physiologic decrease of FSH in a normal cycle is prevented by administration of FSH in the late follicular phase, the augmented sensitivity for FSH allows several follicles to gain dominance ( Fig. 30.11 ). As demonstrated previously in the monkey model, when the negative feedback effect of E 2 on gonadotropin production is suppressed by administration of antiestrogens, selection of the preovulatory follicle is overridden. Further studies regarding the process of selection of the dominant follicle in the normal cycle have indicated that throughout the cycle up to 10 nondominant follicles (measuring between 2 and 10 mm in diameter) can be visualized by transvaginal ultrasound. The dominant follicle itself can be identified once it has reached a diameter beyond 9 mm. Endocrine studies have confirmed that E 2 levels in the serum and follicular fluid begin to rise only after a dominant follicle is present. The abovementioned initial research findings provided the theoretical basis for developing and monitoring a step-down regimen of ovulation induction.
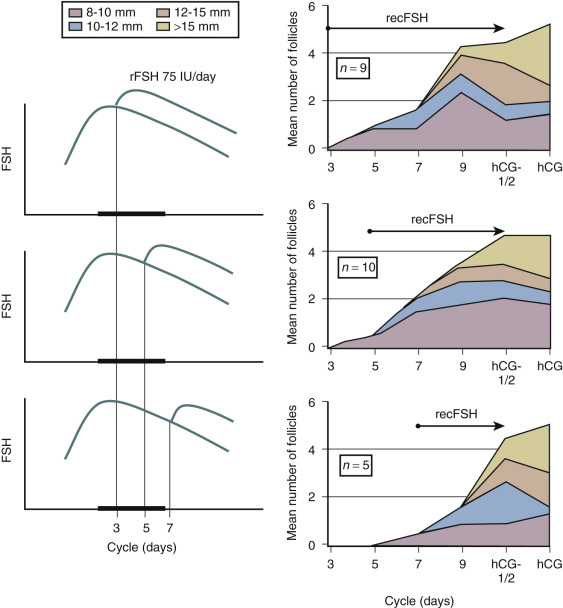
Clinical Outcomes
In what remains one of the largest series describing outcomes using the low-dose step-up regimen, 225 women with PCOS, with ovulation and pregnancy rates of 72% and 45%, respectively, were reported. Studies focusing on further reducing the starting dose have reported the feasibility of commencing with 50 IU or 37.5 IU.
In a randomized trial comparing outcomes following the low-dose step-up versus step-down protocol, the clinical benefits of a more physiologic means of stimulating follicle development were reflected in an incidence of monofollicular cycles of 88% compared to 56% observed in women treated with the step-up regimen, presumably reducing the risk of multiple pregnancy and hyperstimulation.
The degree to which the type of FSH compound employed may influence outcomes in ovulation induction continues to be subject of some controversy. Two meta-analyses comparing the effectiveness of daily uFSH to daily hMG for inducing ovulation in women with PCOS who had not responded to CC demonstrated no difference in pregnancy rate per treatment cycle. However, the women given FSH were less likely to have moderately severe or severe OHSS. The total dose of recFSH needed and duration of treatment was less, and the complication rates were similar. In a later meta-analysis of RCTs comparing recFSH with uFSH for ovulation induction in women with CC-resistant PCOS, no significant differences were demonstrated for the ovulation rate (OR 1.19; 95% CI, 0.78 to 1.8). Furthermore, the odds ratios for pregnancy rate (OR 0.95; 95% CI, 0.64 to 1.41), miscarriage rate (OR 1.26; 95% CI, 0.59 to 2.7), multiple pregnancy rate (OR 0.44; 95% CI, 0.16 to 1.21), and OHSS (OR 1.55; 95% CI, 0.5 to 4.84) showed no significant difference between recFSH and uFSH. In terms of cost-effectiveness, a recent randomized study showed that a lower total dose of recFSH than highly purified urinary FSH was required to achieve the same outcomes. This translated into a 9.4% cost reduction in favor of recFSH.
The success in early clinical studies of pure FSH preparations, increasingly devoid of LH, has served to enhance the impression that excess LH is detrimental to oocyte development and chances of pregnancy following therapeutic intervention. However, a number of recent clinical studies, together with an increasing understanding of the function played by LH in oocyte maturation, have begun to redefine the role of LH as a therapeutic agent in anovulatory fertility. In normogonadotropic anovulation, endogenous LH does not normally require supplementation. Indeed, the focus on LH in this group of patients has been primarily directed at reducing the potential detrimental effects associated with excessive LH. More recently, however, the demonstration of the importance of late follicular LH in optimizing dominant follicle development oocyte quality has reopened the debate as to the role of LH in ovulation induction. Supplementation of LH activity may offer advantages in some patients by hastening large follicle development and therefore shortening the duration of treatment. Moreover, the work of Zeleznik and coworkers referred to a potential therapeutic role for LH in effecting monofollicular stimulation as part of a sequential ovarian stimulation protocol following initiation with recFSH. This concept has been supported in a study in which anovulatory women with a hyperresponse to recFSH were randomized to continue treatment with the addition of either placebo or recLH. In those in whom LH was administered, a trend toward fewer preovulatory follicles was observed. As the availability of recombinant gonadotropins leads to increasing knowledge of the processes of follicular development and selection, further refinements in the efficacy and safety of ovulation induction are likely.
Adverse Effects and Complications
The complications of ovulation induction with gonadotropins are primarily related to excessive ovarian stimulation. Although the aim of therapy is monofollicular growth, multiple follicular developments may occur, causing symptoms of OHSS. Moreover, the development of multiple follicles raises the real risk of multiple pregnancies. To increase the chance of therapeutic success and reduce the risks of complications, careful monitoring of treatment is required. Ovarian response to gonadotropin therapy is monitored using ultrasound to measure follicular diameter. The scans, usually performed every 2 or 4 days, should be focused on identifying follicles of intermediate size; hCG (5000 to 10,000 IU subcutaneously or intramuscularly) is given on the day that at least one follicle measures more than 18 mm. If more than three follicles larger than 15 mm are present, stimulation should be stopped, hCG withheld, and use of a barrier contraceptive advised to prevent multiple pregnancies and OHSS. Measurements of serum E 2 may also be useful. Ovarian stimulation with gonadotropins has not been shown to be associated with long-term risks. Urinary derived FSH is associated with a theoretical risk of transmission of prion proteins. However, the risk of infection is considered to be minimal and not in itself a reason to prescribe recFSH over uFSH.
As confirmed recently, high cumulative singleton live birth rates of almost 80% can be achieved using CC as first line and low-dose FSH as second-line ovulation induction treatment in PCOS, with 14% multiple pregnancy rates ( Fig. 30.12 ). PCOS women with a poor prognosis for ovulation induction, in whom alternative treatment strategies such as IVF may be considered, can best be identified by age, duration of infertility, and body weight.
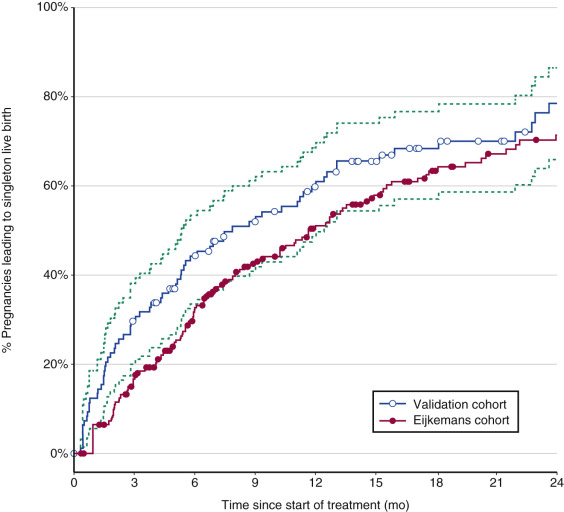
Pulsatile Gonadotropin-Releasing Hormone
Background
In the normoovulatory woman, the pattern of GnRH pulse stimulation alters with the phase of the menstrual cycle, effecting differential gonadotropin synthesis and secretion. During the luteal-follicular transition, pulses occur every 90 to 120 minutes. This slow pulse frequency, in the presence of low E 2 and inhibin A levels, favors FSH production. In the midfollicular or late follicular phase, GnRH frequency increases, favoring LH secretion. In the luteal phase, the production of progesterone increases hypothalamic opioid activity, thus slowing GnRH pulse secretion. This again favors FSH secretion in the luteofollicular transition.
The application of pulsatile GnRH therapy has been demonstrated to be an effective, reliable, and safe alternative to gonadotropin therapy for treating this form of anovulation. Due to the intact ovarian-pituitary feedback system during pulsatile GnRH treatment, the resulting serum FSH and LH concentrations remain within the normal range, and the chances of multifollicular development and ovarian hyperstimulation are therefore low. Little ovarian response monitoring is therefore needed during treatment.
The intravenous route appears superior to the subcutaneous route. To mimic the normal pulsatile release of GnRH, a pulse interval of 60 to 90 minutes is used with a dose of 2.5 to 10 µg per pulse. The lower dose should be used initially to minimize the likelihood of multiple pregnancies. The dose should then be increased to the minimum dose required to induce ovulation. Pulsatile GnRH administration may be continued throughout the luteal phase until menses or a positive pregnancy test. Alternatively, it may be discontinued after ovulation, and the corpus luteum supported by hCG.
Clinical Outcomes
Pulsatile GnRH administration is primarily indicated for women with hypogonadotropic hypogonadal anovulation (WHO type 1) who have normal pituitary function. In these patients, cumulative pregnancy rates of 83% to 95% after six cycles have been reported, with multiple pregnancies accounting for 3% to 8% of pregnancies. Lower ovulation and pregnancy rates have been observed in women with WHO type 2 anovulation, including PCOS. This may be because anovulation in PCOS in part reflects the effects of a persistent, rapid frequency of GnRH stimulation of the pituitary, causing increased LH and T levels. In a recent meta-analysis of four trials comparing pulsatile GnRH with gonadotropins for ovulation induction in women with PCOS, the small size and short follow-up of the studies meant that the authors were unable to draw conclusions on their relative effectiveness.
Regular menstruation occurring approximately every 4 weeks indicates that the woman is having ovulatory cycles. Ultrasonography and measurements of serum progesterone are not usually needed for monitoring therapy. Local complications such as phlebitis may occasionally be encountered when intravenous administration is used. To avoid this, pulsatile GnRH can be administered subcutaneously. This route is certainly simpler than the intravenous approach. However, pharmacokinetic studies comparing the two routes have shown that the plasma GnRH profiles are damped after subcutaneous administration and that bioavailability is reduced. However, the increased convenience offered by the subcutaneous route has led to this approach being favored by many. Smaller devices and more patient friendly delivery systems continue to be developed.
Opioid Antagonists
Background
Endogenous opioid peptides have been shown to play an important role in regulating the pulsatile secretion of gonadotropins by inhibiting the hypothalamic pulse generator that directs GnRH secretion. Infusion of the opiate receptor antagonist naloxone was shown to increase serum LH levels when administered during the late follicular and luteal phase of the cycle.
Clinical Outcomes
Several groups have used naltrexone, an orally active opioid receptor antagonist, to treat ovulatory disorders, with varying degrees of success. The earlier observation that gonadal steroids enhance opioid modulation of gonadotropin secretion was postulated to explain the inability of two groups to demonstrate an increase in gonadotropin secretion or resumption of ovulation in women with WHO class 1 anovulation. However, others have observed restoration of the menstrual cycle. In an uncontrolled study, 19 of 22 women with CC-resistant anovulation were observed to become ovulatory under naltrexone treatment (sometimes in combination with CC) with 12 conceiving. Treatment with 25 mg twice daily was commenced on the first day of a spontaneous or progesterone-induced cycle and continued until a positive pregnancy test occurred or, if no response was observed, for 21 days of treatment. Others have employed doses of up to 100 mg/day. However, conclusions as to the efficacy, optimal regimen, and safety of opiate antagonists for inducing ovulation cannot yet be made. No randomized controlled studies demonstrating their value for this condition have yet been published, and opiate antagonists remain at best a second-line, alternative therapy.
Dopamine Agonists
These agents are primarily used in the treatment of anovulation secondary to hyperprolactinemia. The treatment of hyperprolactinemia and the agents available for treatment are covered in detail elsewhere (see Chapter 3 ).
Kisspeptin
Drugs intervening with the pre-GnRH kisspeptin system are currently being tested for their potential use for both ovarian stimulation and ovarian suppression.
Adjunctive Therapies
Dexamethasone
Glucocorticoids have been proposed as a useful adjuvant to both CC and gonadotropin ovulation induction in women with PCOS with a therapeutic rationale based on reducing ovarian androgen levels, improving ovulatory function, and reducing resistance to ovulation induction agents. Although the source of high androgen secretion in anovulatory women with PCOS is primarily ovarian, 50% to 70% also demonstrate excessive adrenal androgen levels.
To normalize (without suppressing) adrenal steroid production, daily oral doses of dexamethasone (0.25 to 0.5 mg) or prednisone (5 to 10 mg) have been employed in a continuous regimen. Although widely used, the value of adjuvant corticosteroid administration with CC or gonadotropins for ovulation induction remains questionable. In a study of women with PCOS, the chance of ovulation after glucocorticoid suppression of adrenal androgens was not predicted by either basal dehydroepiandrosterone sulfate (DHEAS) levels or suppressed levels, and limited effects on ovulation were observed. A randomized controlled study in 80 women with CC resistance and normal serum DHEAS levels showed significantly higher ovulation and pregnancy rates when 2 mg/day dexamethasone was added from cycle day 2 to 12 to CC 100 mg.
While major complications from the adjuvant use of low-dose glucocorticoids are rare, weight gain is a common problem. Other reported side effects include glucose intolerance and osteoporosis. Given possible side effects, their use should remain as a second-line therapy subject to further research.
Gonadotropin-Releasing Hormone Agonists
Adjuvant GnRH agonist treatment has also been proposed to improve outcomes and reduce complications of ovulation induction. Early uncontrolled studies indicated that the concomitant use of GnRH agonist with ovarian stimulation regimens in women with PCOS was safe and improved treatment outcome. Further studies indicated that premature luteinization could be prevented by employing GnRH agonists, but no clear difference in pregnancy rates was demonstrated. Although a meta-analysis of five prospective studies suggested that improved pregnancy rates could be achieved at similar ovulation rates when GnRH agonists were also employed, a later systematic review concluded that GnRH agonist as an adjunct to FSH and hMG does not improve pregnancy and OHSS rates and should therefore not be recommended as a standard treatment for this patient group.
Conflicting data on the effects on ovulation and pregnancy rates, combined with reports of severe OHSS with adjuvant GnRH agonist therapy and the additional burden for the patient of prolonged treatment cycles, mean that adjuvant GnRH agonists remain a second-line therapy in conjunction with FSH stimulation.
The availability of GnRH antagonists provides new opportunities to modify ovulation induction regimens. Particular attributes of GnRH antagonists that might be of value in this context include their competitive binding properties, immediate suppression of the pituitary without a flare-up effect, and rapid resumption of gonadal function on discontinuation. However, few studies have appeared which further explore its role in this clinical context.
Additional Treatable Factors Influencing the Balance of Efficacy and Risks
Obesity
Among women with WHO class 2 anovulation, obesity may be present in up to 50%. In addition to enhancing the features of insulin resistance mentioned earlier, overweight (BMI >32) is also associated with reproductive dysfunction despite regular menstrual cycles. In recent years, considerable attention has been given to the role of lifestyle factors and management in improving outcomes in obese anovulatory women. Even a small (2% to 5%) reduction in weight has been shown to improve metabolical indices including insulin resistance. In addition, weight loss can lead to a rise in sex hormone–binding globulin (SHBG) concentrations, a decrease in FAI and T levels, and improvement in cyclicity. A relatively modest reduction in weight has been shown to increase the frequency of ovulation in obese anovulatory women to more than 70%. Energy restriction acting to temporarily improve insulin sensitivity may be important, because improvements in endocrine and clinical parameters occurred maximally during the period of energy restriction. During subsequent weight maintenance, many benefits were reversed.
The evidence for the benefits of weight loss combined with recent data confirming BMI to be a major factor influencing outcome of ovulation induction make the treatment of obesity an important adjuvant treatment that should precede ovulation induction. Given the baseline risks of ovulation induction and the possible risks of obesity for subsequent pregnancy and general health, weight loss in cases of obesity should be considered as a prerequisite to medical ovulation induction treatment.
Tobacco Smoking
Epidemiologic data provide strong evidence for a causal association between cigarette smoking and other lifestyle factors and decreased fertility. For a recent review of the impact of smoking and other lifestyle factors on fertility treatment outcomes, see Homan and colleagues. Dose-dependent effects of smoking have been reported in relation to the duration to conception. Moreover, there is evidence of increased risk of early pregnancy loss in smokers and a reduced mean age at menopause. Although properly designed studies of the effect of smoking on outcomes of ovulation induction are scarce, data from studies in assisted conception point to detrimental effects on ovarian function and oocyte quality, which are likely to be applicable to the situation concerning ovulation induction. In any discussion of infertility therapy, the clinician should emphasize the risks of smoking for outcome of treatment. Indeed, preconceptional care and lifestyle advice should be an integral part of the modern fertility clinic.
Ovarian Stimulation in the Empirical Treatment of Unexplained Subfertility
- ◆
Despite numerous studies, the added value of ovarian stimulation for the empirical treatment of unexplained infertility remains uncertain, especially in the context of multiple pregnancy related cost and complications.
- ◆
Empirical ovarian stimulation is often combined with intrauterine insemination, although both interventions can be used separately.
Principles of Ovarian Stimulation
The aim of ovarian stimulation is to intervene in the mechanisms regulating single dominant follicle selection to mature multiple follicles and obtain multiple oocytes for fertilization in vivo (either after timed intercourse or IUI) or IVF. Ovarian stimulation is usually performed in normoovulatory infertile women to increase chances for pregnancy. However, the development of multiple follicles inherently also increases the undesired risk of (higher order) multiple pregnancies and OHSS. In IVF, OHSS risks are reduced because of the puncture of all visible large follicles to retrieve the oocytes, and incidences of multiple pregnancies can be controlled by limiting the number of embryos transferred.
Obviously, oligovulatory and anovulatory women may also qualify for either IUI or IVF after failed ovulation induction. Ovarian stimulation may also be performed in these women, aiming at multiple follicle development. It should again be emphasized that this condition of hyperstimulation in these patients is distinctly different from ovulation induction in which the aim is to mimic physiology and stimulate ongoing growth and ovulation of a single dominant follicle. However, these patients are usually difficult to manage because of an unpredictable major individual variability in response and a tendency to hyperrespond to stimulation protocols.
Although daily administration of ovary stimulating agents allows for dose adjustments based on individual ovarian response monitoring, the clinical evidence for the efficacy of such an approach is scant. A hyperresponse may be counteracted by a dose decrease or the complete cessation of exogenous gonadotropins for some days (the latter strategy is referred to as “coasting”). An excessive number of follicles for ovulation induction or hyperstimulation for IUI may be reduced by follicle puncture or cycle cancellation. When in contrast low ovarian response to standard stimulation is observed, recent evidence indicates that a gonadotropin dose increase does not result in improved outcome. This is not surprising if the pathophysiologic background of low response is taken into consideration. Low response to ovarian stimulation may be the first sign of advanced ovarian aging. Women with a previous low response to stimulation have been shown to enter menopause at an earlier age.
During the normal menstrual cycle, the midcycle LH surge represents the trigger for inducing final oocyte maturation, the rupture of the follicle and release of the oocyte, and finally luteinization of granulosa and theca cells allowing for the formation of the corpus luteum. As mentioned before, the synchrony of endocrine events inducing the LH surge is disrupted in ovarian stimulation. Therefore, the endogenous LH surge is replaced by an exogenous hCG bolus injection, timed by the visualization of large graafian follicles upon ultrasound. Finally, these follicular phase interventions result in luteal phase abnormalities requiring luteal phase supplementation by either hCG or exogenous progestins.
Therapeutic Approaches
Unexplained infertility is usually diagnosed by exclusion when standard infertility investigation shows no abnormalities. However, no agreement exists regarding the preferred extent of standard investigation as well as the interpretation and prognostic value of many of these tests. Usually, ovulation is assessed by a midluteal phase serum progesterone assay, tubal patency is established by hysterosalpingogram, and male factor infertility is excluded by semen analysis. Again, the interpretation of any of these tests is not without difficulty, and many clinicians perform additional tests to further explore possible causes of infertility. Hence, the term unexplained infertility is notoriously ambiguous and may mean anything in between undiagnosed infertility and normal fertility in which a pregnancy did not occur merely by chance. This may especially be the case in young women who have been attempting to conceive for a relatively short time.
It should be realized that many biologically relevant processes important for obtaining a pregnancy—such as oocyte chromosomal constitution, subtle sperm abnormalities, in vivo conception, embryo transport and implantation, and finally endometrial receptivity—cannot be studied accurately as yet.
When a couple presents with unexplained infertility, it is extremely important to assess chances of spontaneous pregnancy before commencing on any kind of empirical therapy. As mentioned before, ovarian hyperstimulation (with or without additional interventions such as IUI) may enhance pregnancy chances per cycle, but at the cost of patient stress and discomfort, chances for side effects such as multiple gestation and OHSS, and high costs. Similar cumulative pregnancy rates may be achieved with expectant management for 6 to 12 months. Expectant management may represent the most favorable approach in many young women with a short duration of infertility.
Results are frequently reported from combined interventions such as ovarian stimulation and IUI. These studies are often uncontrolled, and few are sufficiently powered to differentiate between the independent effects of hyperstimulation and IUI and the potential additive effects of combining both interventions. In recent years, the picture has become clearer. Although the absolute treatment effect appears relatively limited, given the low cost and ease of administration, CC can be recommended as first choice medication for the treatment of unexplained infertility. In terms of pure efficacy, however, a meta-analysis of five trials indicated that gonadotropins may be superior to CC as ovarian stimulation agents for the treatment of unexplained infertility. Treatment with CC was associated with significantly reduced odds ratios of pregnancy per woman compared to gonadotropins (OR 0.41; 95% CI, 0.17 to 0.8). As far as complications are concerned, no significant differences could be found for miscarriage (OR 0.61; 95% CI, 0.09 to 4) or multiple birth (OR 1.1; 95% CI, 0.2 to 7). The incidence of OHSS or cycle cancellation rates could not be assessed.
For unexplained infertility, the combination of IUI with ovarian stimulation potentially bypasses several possible barriers to fertility, including minor sperm abnormalities, sperm-cervical mucus interactions, timing of sperm delivery problems, and a possible beneficial effect of ovarian stimulation on endometrial receptivity. The most important benefit is likely to be the stimulation of multiple follicles. Although a meta-analysis by Hughes has addressed questions relating to the benefits of FSH and IUI alone compared with combined therapy, less than a third of the studies included in the analysis make use of treated control subjects. Moreover, the conclusions that both FSH and IUI improve fecundity are derived from regression analysis and are open to discussion. Other studies have indicated that ovarian hyperstimulation with both CC and gonadotropins improve the fecundity rate compared to IUI alone. However, a study comparing intracervical insemination alone with FSH in combination with IUI showed a statistically higher pregnancy rate with the latter treatment combination. The number needed to treat was 31 cycles. This implies that it would take 31 cycles of treatment before there would be one more singleton live birth with FSH and IUI than with intracervical insemination alone. The number needed to treat with FSH in combination with IUI to obtain an extra pregnancy above that obtained with IUI alone is even greater.
When the costs of multiple pregnancies arising from multiple follicle development are considered, the cost effectiveness of FSH and IUI combined therapy for this indication may be limited. Cost-effectiveness analyses have led to the conclusion that IUI with or without stimulation should precede IVF. In clinical practice, the benefits of ovarian stimulation in combination with IUI need to be weighed against the additional discomfort and costs of monitoring applied, often unsuccessfully, to avoid multiple pregnancy. Clearly, more studies are needed to elucidate the optimal approach to treating unexplained infertility and the role ovarian hyperstimulation should play.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree