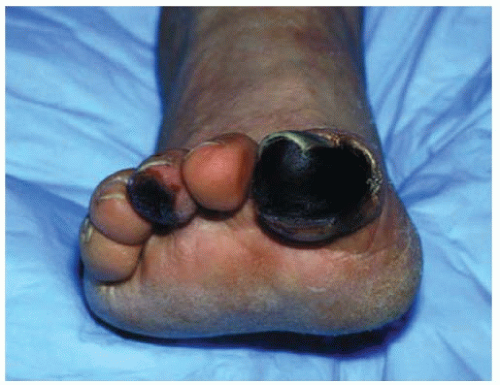
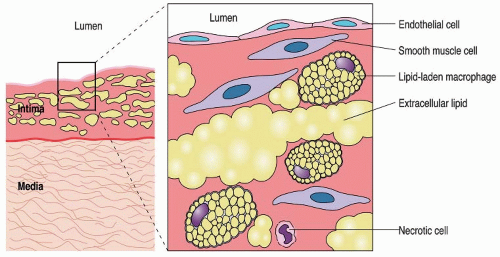
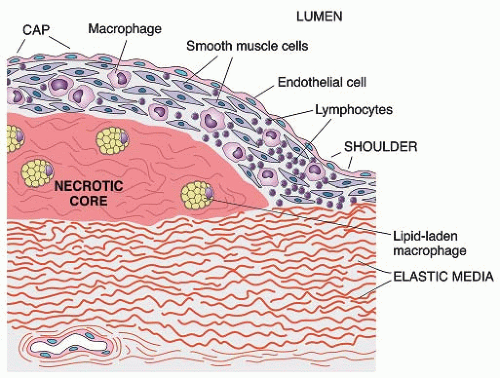
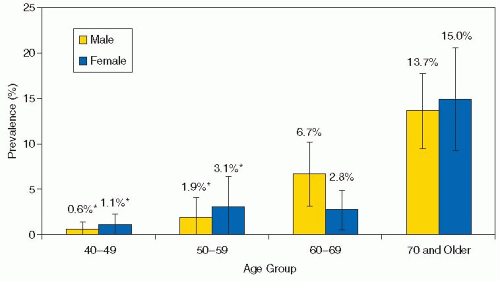
Peripheral arterial disease (PAD) affects approximately 27 million individuals in North America and Europe, and its prevalence is increasing as life expectancy increases.
1 Atherosclerosis is the most common cause of PAD, with symptoms primarily attributable to arterial stenosis and reduced blood flow. Nonatherosclerotic PAD is far less common, resulting from vasculitis, arterial vasospasm, or thromboembolism. Moreover, the development of PAD represents one manifestation of an atherosclerotic process, which in the majority of individuals affects the entire vascular tree. Consequently, patients with PAD are at an increased risk for coronary artery disease and cerebrovascular disease.
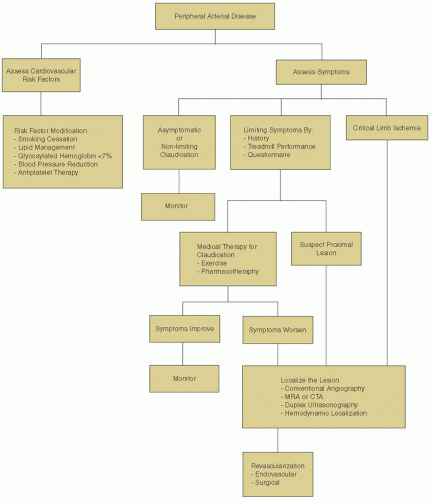
The diagnosis and management of PAD has evolved considerably over the past few decades. Advances have stemmed from improved diagnostic modalities as well as from new medical and surgical treatments, with a greater focus on overall cardiovascular risk reduction. Despite these improvements, the successful treatment of individuals with PAD remains a challenge and encompasses a multifaceted strategy involving lifestyle modification, pharmacologic therapy, and peripheral arterial interventions. This chapter provides an overview of the pathogenesis, epidemiology, and management of PAD related to the lower extremities.
RISK FACTORS FOR THE DEVELOPMENT OF PAD
The risk factors for PAD are the same as those for other atherosclerotic disease processes, modifiable ones including smoking, dyslipidemia, diabetes, and hypertension. In the National Health and Nutrition Examination Survey (NHANES), 95% of patients had at least one modifiable cardiovascular risk factor and 75% of patients had at least two cardiovascular risk factors.
14 Advanced age represents a nonmodifiable risk factor for PAD development.
Smoking causes several deleterious alterations in lipid metabolism, including reduced high-density lipoprotein (HDL) and increased LDL, triglyceride, and total cholesterol levels.
20 In
addition, smoking directly influences atherosclerosis by causing endothelial dysfunction, inflammation, and coagulation within the vascular bed.
21 Individuals who smoke are at risk for cardiovascular events, such as sudden cardiac death, nonfatal myocardial infarction, and ischemic stroke. Active smokers are at a 4.6-fold increased risk of developing PAD compared to nonsmokers,
14 and smoking is associated with a threefold increased risk of CLI, with both PAD severity and progression directly correlated to the number of cigarettes consumed.
Diabetes is a strong modifiable risk factor for PAD, associated with a 2.71-fold increased risk.
14,22 In the Framingham registry, men were at a twofold increased risk for stroke in the presence of PAD and diabetes compared to either disease alone, and women with diabetes and claudication symptoms were at a three- to fourfold increased risk for coronary artery disease, stroke, and heart failure.
22,23 The presence of diabetes signifies a more aggressive course of PAD, with diabetic patients at a 5- to 10-fold increased risk for major limb amputation compared to those without diabetes.
16
Several mechanisms have been proposed to explain the aggressive nature of vascular disease observed in diabetics. Clustering of cardiovascular risk factors, such as hypertension and dyslipidemia, partly account for the increased cardiovascular event rate. Patients with diabetes frequently develop dyslipidemia secondary to insulin resistance, resulting in decreased HDL, with increased LDL and triglycerides.
24 Significant deleterious effects on vascular biology also occur in the setting of insulin resistance and hyperglycemia.
25 Increased oxidative stress and platelet activation occur in the setting of hyperglycemia, placing patients with diabetes at increased risk for vascular thrombotic events. Negative effects of insulin resistance on SMC proliferation and cellular apoptosis promote further atherosclerosis progression. The development of endothelial dysfunction can impair cellular glucose transport, potentially leading to further insulin resistance and worsening diabetes.
26 Clinical studies support the causal relationship of hyperglycemia, insulin resistance, and accelerated atherosclerosis, as reflected by a direct correlation of cardiovascular disease with glycosylated hemoglobin levels in diabetic populations.
27Dyslipidemia: Patients with PAD demonstrate significant abnormalities in lipid metabolism; however, the associated risk appears to be lower compared to other vascular risk factors such as diabetes or smoking. In the NHANES registry, hypercholesterolemia (defined as a total cholesterol level 240 mg/dL [6.2 mmol/L]) was associated with a 1.68-fold increase in the prevalence of PAD.
14 Abnormalities in total cholesterol, LDL, very low-density lipoprotein, and lipoprotein (a) levels are associated with PAD, while serum triglyceride levels are only modestly associated with PAD.
28,29
Hypertension results in a 1.5- to 2-fold relative risk (RR) increase for PAD.
14 Hypertension significantly alters vascular through increased endothelial dysfunction, loss of precapillary networks, and increased microvascular vasoconstriction in hypertensive animal models; all of which beget further increases in blood pressure, promoting both atherosclerosis and thrombosis.
Advanced age represents a risk factor for cardiovascular events and worse clinical outcomes in patients with established PAD. In the SMART registry of patients with symptomatic PAD, each 10-year increase in age was associated with a 1.85-fold risk increase in fatal and nonfatal vascular events.
30
Genetic risk: The genetic contribution to PAD has not been clarified to the same extent as in other cardiovascular diseases. Several family studies suggest that PAD is in part related to genetic risk, estimating that genetic variation accounts for 21% to 48% of the interindividual variability in the ABI.
31 Genome-wide association studies have enabled further insight into the role of common genetic variants in cardiovascular disease. The genetic epidemiology network of arteriopathy study demonstrated that PAD (identified as a reduction in the ABI) was modestly heritable in African American and white hypertensive populations. Single-nucleotide polymorphisms (SNPs) associated with PAD were observed in several loci, but no single SNP reached genome-wide significance (
P ≤ 5 × 10
-8).
32 A common variation of the chromosome 9p21 allele has been associated with several forms of vascular disease, particularly myocardial infarction and stroke.
33,34,35,36 In elderly individuals, Cluett et al.
37 demonstrated a modest association between the 9p21 allele and PAD detected by ABI (odds ratio [OR] 1.29, 95% CI 1.06 to 1.56). Further studies are necessary to identify common genetic variants associated with PAD development and to clarify the role of genetic testing in risk stratification of PAD patients.