Mediastinal and Tracheal Cancer
 MEDIASTINAL TUMORS
MEDIASTINAL TUMORS
Mediastinal malignancies are quite heterogenous in scope. Invasive thymomas and thymic carcinomas are relatively rare tumors, together representing about 0.2% to 1.5% of all malignancies.1 Thymic carcinomas are rare, accounting for only 0.06% of all thymic neoplasms.2 Arising from thymic, neurogenic, lymphatic, germinal, and mesenchymal tissues, mediastinal tumors are usually located in the anterior mediastinum but can also appear in the posterior and middle mediastinum or neck. Lymphomas, the most common type of mediastinal tumor, are discussed in detail in Chapter 89.
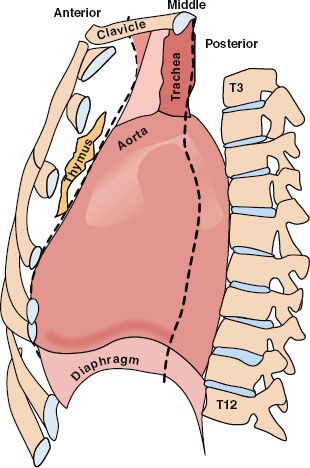
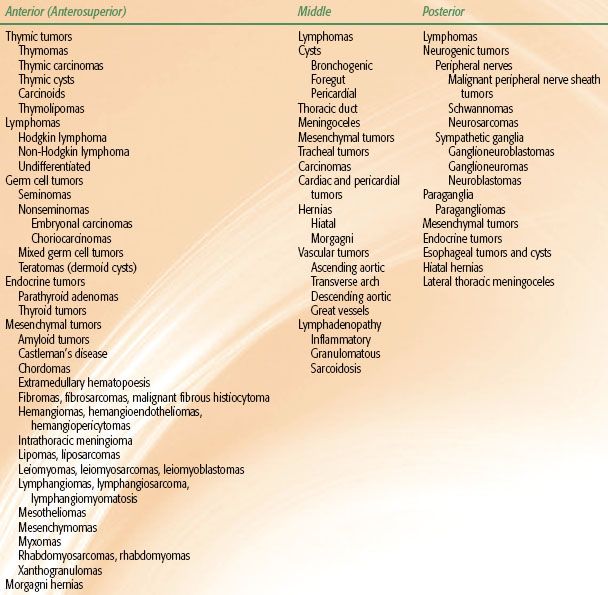
Anatomically, the mediastinum is trapezoidal in shape and is essentially the center of the thoracic cavity. It extends from the sternum anteriorly to the vertebral column posteriorly. The lungs and parietal pleurae are the lateral borders, the diaphragm is the floor, and the thoracic outlet of T1, its rib, and the manubrium form the roof of the mediastinum.3 Conceptually, the mediastinum can be subdivided into three compartments (Fig. 52.1), but no anatomic barriers physically separate the compartments.4–5,6 The anterior mediastinum is the space anterior to the pericardium and great vessels and is occupied by the thymus, lymph nodes, and small vessels. The middle mediastinum is comprised of the heart, proximal great vessels, central airway structures, and lymph nodes. Tracheal anatomy is depicted in Figure 52.2. The posterior mediastinum is posterior to the heart and great vessels and contains the sympathetic chain ganglia, vagus nerve, thoracic duct, and esophagus. Neoplastic masses that occur within the various compartments in the mediastinum are listed in Table 52.1.
Incidence of Primary Mediastinal Tumors
The frequency and prevalence of primary mediastinal tumors seem to be increasing over time.7–10 Adults generally develop thymic tumors and lymphomas, but they can also develop germ cell tumors and carcinomas.10,11 Neurogenic tumors are usually seen in children.8,12,13
FIGURE 52.1. Anatomy of the mediastinum.
 THYMOMAS
THYMOMAS
The thymus gland is an irregular lobulated lymphoepithelial organ in the anterior mediastinum. Embryologically, the thymus is derived from the endoderm of the lower portion of the third pharyngeal pouch and involutes during adulthood, gradually being replaced by adipose tissue. The blood supply is from the internal mammary arteries. The venous drainage is to the innominate and internal thoracic veins. The lymphatics drain into the lower cervical, internal mammary, and hilar nodes.
The vast majority of thymic tumors are thymomas, 90% of which are found in the anterosuperior mediastinum; other variants occur in the middle and posterior mediastinum or neck.14 Thymomas are epithelial tumors associated with an exuberant lymphoid component composed of immature cortical thymocytes. Although lymphomas, carcinoid tumors, and germ cell tumors can all arise within the thymus, only thymomas, thymic carcinomas, and thymolipomas arise from true thymic elements.
FIGURE 52.2. Representative schema of the trachea. Note the lateral longitudinal anastomotic artery running parallel to the organ and the intercartilaginous branches feeding each tracheal segment. The trachea resides in close proximity to the esophagus.
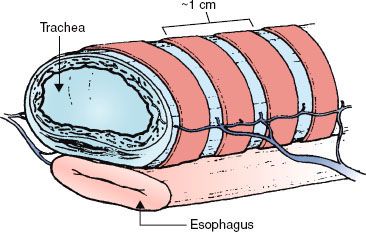
TABLE 52.1 HISTOLOGIES OF TUMORS APPEARING IN THE VARIOUS COMPARTMENTS OF THE MEDIASTINUM
Epidemiology
Thymomas are exceedingly rare. The Surveillance, Epidemiology, and End Results (SEER) project reported the thymoma incidence to be 0.15 per 100,000 person-years.15 For patients with associated myasthenia gravis, the peak age is in the fourth decade, whereas for patients without myasthenia gravis, the peak age is in the seventh decade or later.16,17–18,19–21 According to the SEER data, the incidence of thymoma increases into the eighth decade of age and then decreases.15 Thymomas are more common in men than in women (P = .007) and are most common among Asians or Pacific Islanders (0.49 per 100,000 person-years).
Thymomas are the most common of the anterior mediastinal masses, accounting for about 30% of all such masses.8,9–10,14,22,23 Of all mediastinal masses, thymomas represent 20% of the tumors in adults6,11,24,25 and 15% in pediatric populations.14 Associations of thymomas with Epstein-Barr virus, lymphoepitheliomas, radiation exposure, and cytogenetic abnormalities have been suggested.26–32
Natural History
Thymomas are generally characterized by an indolent growth pattern that can be locally invasive. Thirty percent to 40% of patients with a thymoma also have myasthenia gravis.33 The vast majority of thymomas are cytologically bland tumors and approximately half of them are noninvasive.10,21,34–36,37,38–44 Roughly one-third of thymomas are asymptomatic and found incidentally on chest x-rays.40,41,45 Of the symptomatic thymomas, about 40% of cases present with symptoms relating to impingement by the intrathoracic mass, ranging from cough, chest pain, dyspnea, hoarseness, superior vena cava obstruction, and even tumor hemorrhage.46 Another 30% present with systemic signs and the remainder present with signs of myasthenia gravis.
Thymomas are associated with several parathymic syndromes, the most common of which is myasthenia gravis47,48; other autoimmune conditions such as benign cytopenia, hypogammaglobulinemia, and polymyositis have been reported in 2% to 5% of patients.16,19,47,48–50 Myasthenia gravis is characterized by the presence of antibodies that react with nicotinic acetylcholine receptors in muscle and disrupt transmission at the neuromuscular junction.51 The cardinal features are weakness and fatigability of skeletal muscles, with most patients first experiencing fatigue in the ocular muscles followed by ptosis and diplopia and later developing generalized weakness. More severe cases involve the proximal limb girdle muscles and, in the worst cases, can even affect respiration.52–53,54 Myasthenia gravis occurs in approximately 45% of patients with thymomas, with the reported prevalence in studies involving more than 100 patients ranging from 10% to 67%.17,18,20,21,55,56 Conversely, only 10% to 15% of patients with myasthenia gravis have a thymoma.18,51,57 Roughly one-fourth of patients with myasthenia gravis will have a normal thymus.58 Of the 75% who have an abnormal thymus, only 15% to 20% will have a thymoma and 60% will have thymic lymphoid hyperplasia.59 Thymectomy results in clinical improvement in most cases even when the thymus is normal.60
Other systemic symptoms occur in 5% to 10% of patients with thymomas as part of a constellation of autoimmune disorders. Souadjian et al.,47 in reviewing more than 500 cases of thymoma, noted that 71% were associated with systemic disease. These include erythroid and neutrophil hypoplasia, pancytopenia, Cushing syndrome, DiGeorge syndrome, carcinoid syndrome, Lambert-Eaton syndrome, pernicious anemia, nephrotic syndrome, syndrome of inappropriate antidiuretic hormone hypersecretion, Whipple’s disease, lupus erythematosus, pemphigus, myotonic dystrophy, scleroderma, polymyositis, polyneuritis, myocarditis polyarthropathy, myotonic dystrophy, Sjogren syndrome, Addison’s disease, panhypopituitarism, sarcoidosis, hypogammaglobulinemia, ulcerative colitis, rheumatoid arthritis, Hashimoto’s thyroiditis, hyperthyroidism, hyperparathyroidism, and thyroid carcinoma.14,61–63,64 Other miscellaneous diseases include hypertrophic osteoarthropathy and chronic mucocutaneous candidiasis.63
Several studies have also reported an average excess risk of developing a second primary malignancy of 15% for patients with thymomas over that expected in the normal population.47,49,50,65–67 In one study, the most notable excess risk of subsequent malignancy was for non-Hodgkin lymphoma, with digestive system and soft tissue sarcomas being elevated as well.15
The vast majority of thymomas are indolent, but if the tumors spread, they most commonly implant on regional pleural surfaces and can cause pleural plaques, diaphragmatic masses, and malignant pleural effusions.16 The rate of lymphogenous metastasis in one of the largest databases of 1,093 patients with thymomas was 1.8%, with 90% of positive lymph nodes located in the anterior mediastinum.68 Only rarely do thymomas spread hematogenously, but metastases have been reported in the liver, lung, and bone.69
Diagnosis
Thymic tumors account for 50% of all anterior mediastinal masses, another 25% are lymphomas, and the remainder are various other tumors (see Table 52.1).10 The latter group often has characteristic radiographic findings (e.g., teratomas). Lymphomas often have other suggestive systemic symptoms or clinical findings such as weight loss, fevers, and lymphadenopathy.
Biopsy can be performed via a fine-needle aspiration, bronchoscopy, mediastinoscopy, video-assisted thoracoscopy, or open biopsy. Often a clinical diagnosis is sufficient for a small thymoma in a patient with a parathymic syndrome. Traditionally, biopsies were avoided because of concern about tumor spillage into the pleural space when the capsule was breached.70,71 However, no cases of seeding of a needle tract or the biopsy site have been reported, and only three recurrences in thoracotomy scars have been reported.72,73 A multivariate analysis in one series of 136 patients being treated for thymomas showed better survival among patients who underwent biopsy before surgery (P = .056).50 Many centers routinely obtain biopsy samples of larger tumors.18,49,72,74,75–77
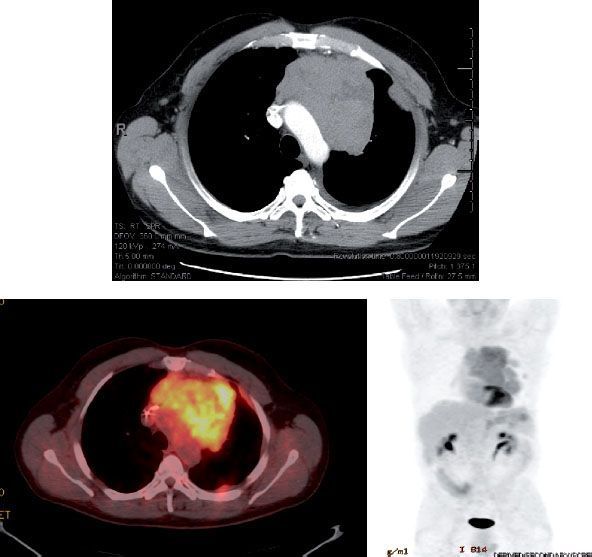
The diagnostic workup begins with a careful evaluation for myasthenia gravis. Routine blood work for common associated syndromes should be done, with serum α-fetoprotein and β-human chorionic gonadotropin in men to rule out a germ cell tumor.78 Computed tomography (CT) often allows visualization of an anterior mediastinal mass.43,79–81 Magnetic resonance imaging (MRI) can provide more detail when needed, delineating the musculoskeletal anatomy and neurovascular structures of the mediastinum.82–83,84 The use of 18-fluorodeoxyglucose (18F) positron emission tomography (PET) is expanding for visualizing several types of malignancies, including thymoma. However, results of PET can be confounded by the physiologic uptake of fluorodeoxyglucose by the thymus in children and young adults.85,86,87 Nevertheless, this imaging modality has continued to show promise for disease detection and for evaluating prognosis. Recent studies have shown that not only can PET improve the sensitivity of diagnosis, but it also can be helpful for establishing the grade of the disease.88–89,90,91 In one provocative study in which 49 patients with thymic tumors underwent PET imaging, the rate of fluorodeoxyglucose uptake was compared with the expression of several biologic markers, including glucose transporter-1 (GLUT-1), GLUT-3, hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), microvessel density, CD31 and CD34, p53, and B-cell lymphoma-2 (bcl-2). The authors found a direct correlation between fluorodeoxyglucose uptake on PET and expression of GLUT1, HIF-1α, VEGF, and p53 as well as microvessel density, and several of these markers also correlated with tumor grade.92 These findings are important because they imply that treatment efficacy could be monitored in terms of metabolic response. Figure 52.3 demonstrates PET findings from a patient with locally advanced thymoma treated with trimodality therapy. Imaging such as this, in conjunction with contrast-enhanced CT scans of the chest, clearly can help in precisely delineating the extent of disease.
In addition to PET, octreotide scanning has shown some efficacy for diagnosing thymic malignancies in small series.93 MRI was also found to offer improved sensitivity and to provide information regarding tumor grade and invasiveness beyond that which can be gleaned from CT. Common features indicative of a high-grade tumor include low T2-signal foci within the mass, the presence of mediastinal lymphadenopathy, an incomplete capsule, and inhomogenous enhancement.94,95
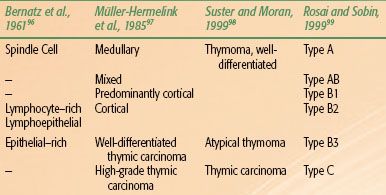
TABLE 52.2 THYMOMA CLASSIFICATION SYSTEMS
Pathologic Classification
Thymomas have been extensively studied by pathologists because of their varied appearance and the frequent lack of classic malignant features. Several classification systems have been proposed and are in use (Table 52.2). Indeed, even the terminology used to describe thymomas has been inconsistent and imprecise. Thymomas have most often been considered invasive versus noninvasive or alternatively “benign” versus malignant. However, recurrences and metastases after resection have been reported in all large series,17,18,100–102 regardless of disease stage or histologic subtype.16,17–18,20,21,42,49,50,101–102,103,104–105,106,107–109 Even bland-appearing, noninvasive thymomas have the fundamental characteristics of a malignant tumor in their ability to recur and metastasize.
FIGURE 52.3. Positron emission tomography (PET) scanning in thymic malignancies. PET imaging has been shown to aid in delineating the extent of disease and assessing for metastases.
The most recent classification was proposed in 1999 and updated in 2004 by the World Health Organization (WHO).99,110 However, a historical perspective of thymoma classification systems is necessary to interpret the literature. The earliest system, proposed by Bernatz et al.,96 identified four categories based on predominant cell type: lymphocytic, epithelial, mixed, and spindle cell. The predominant problem with this classification system, as is true of all systems, is its poor correlation with clinical prognosis. Verley and Hollmann101 also identified four categories: spindle or oval, lymphocyte-rich, differentiated epithelial cell–rich, and undifferentiated epithelial. The first three types are cytologically bland, whereas the fourth category includes pleomorphic tumors with high numbers of mitoses and atypia, which are also considered thymic carcinoma. Unfortunately, this categorization also does not correlate well with prognosis.
Müller-Hermelink et al.97 based their classification system on the fact that the thymus consists of different subsets of epithelial cells: cortical, medullary, mixed cortical and medullary, and well-differentiated. Although this has been the most widely used system, little association has been found between epithelial cell morphology and prognosis.111 Although some associations have been found, such as cortical thymomas behaving more aggressively and being more often associated with myasthenia gravis,75,101,105,112–114 the relationship between histologic types and prognosis is not consistent.19,76,111,115 Several multivariate analyses of large numbers of patients have shown that the Müller-Hermelink classification system is not an independent predictor of survival.16,19,74
Thymic carcinomas can be readily subclassified into well or poorly differentiated. Well-differentiated thymic carcinomas have features typical of thymomas but also contain areas of atypia and mitoses, but usually fewer than 2 per 10 high-power field.116 The incidence of these tumors and the prognosis associated with them vary among studies. Poorly differentiated thymic carcinomas are clearly recognized as a distinct group. The virtual absence of parathymic syndromes and clear-cut cellular atypia are consistently associated with poor prognosis. Thymic carcinomas can be subdivided into squamous cell, mucoepidermoid, basaloid, lymphoepithelioma-like, small cell or neuroendocrine, sarcomatoid, clear cell, and undifferentiated or anaplastic subtypes.117 Thymic carcinoid is sometimes referred to as a neuroendocrine thymic tumor because of the presence of neuroendocrine granules, but thymic carcinomas can also contain these granules.
Another problem confounding the use of these classification schemes is that they do not consistently correlate with other schemes.111 Concordance rates for thymomas classified independently by a panel of pathologists were often as low as 35% within one system, although two small studies demonstrated 78% concordance when using the Müller-Hermelink classification system.118,119 Considering the persistent problem of the poor correlation between the histopathology of thymomas and their malignant potential and the lack of correlation with systemic syndromes,43 the demand for a more consistent classification is obvious.
The WHO classification is similar to the Müller-Hermelink system but recognizes six different types of thymic tumors (A, AB, B1, B2, B3, C; see Table 52.2).110,120 Type A tumors are composed of neoplastic oval or spindle-shaped epithelial cells without atypia or lymphocytes. Type AB is similar to type A, but with foci of lymphocytes. Type B tumors consist of plump epithelioid cells that can be subdivided into three subtypes defined by increasing proportions of epithelial cells and increasing atypia. Type B1 tumors resemble normal thymic cortex with areas similar to thymic medulla. Type B2 have scattered neoplastic epithelial cells with vesicular nuclei and distinct nucleoli among a heavy population of lymphocytes; perivascular spaces are prominent and a palisading effect of tumor cells along the perivascular spaces may be present. Type B3 is composed of predominantly round or polygonal epithelial cells exhibiting mild atypia admixed with a minor component of lymphocytes; thus, this type resembles what others have described as well-differentiated thymic carcinoma. Thymic carcinomas are designated type C tumors and have clear-cut cytologic atypia and a cytoarchitecture resembling carcinoma that is distinctively unlike normal thymus tissue.116
TABLE 52.3 MASAOKA STAGING SYSTEM FOR THYMOMAS

TABLE 52.4 GROUPE D’ETUDES DES TUMEURS THYMIQUESTHYMOMA STAGING SYSTEM
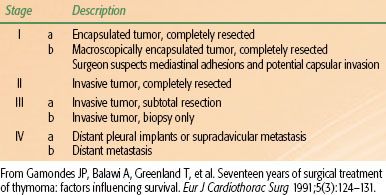
The WHO classification has been evaluated by multiple groups since it was proposed. Concordance rates among different pathologists using the WHO system were 90% and 95% in two studies.121,122 However, comparison of the WHO subtypes across studies demonstrated that the varying incidence of A and B subtypes was greater than could be ascribed to chance alone.116 The most clinically distinct subtypes are type B3, formerly known as well-differentiated thymic carcinoma, and type C, thymic carcinomas. The clinical characteristics associated with the other WHO subtypes still vary considerably, but the WHO system seems to correlate well with Masaoka stage (Table 52.3) in studies in which patients were analyzed with the WHO system.116 The vast majority of type A and AB tumors are Masaoka stage I or II, and the B subtypes tend to resemble the higher Masaoka stages.116
The WHO system has been found to have independent prognostic value for disease-specific survival, with only two small studies indicating that stage was the only prognostic variable.116 Most of the prognostic value of the WHO classification is attributable to type C, having distinctly worse survival,123–125 but even studies that excluded type C could demonstrate WHO type as having independent prognostic value.125,126
More recent pathologic studies have provided intriguing insights on prognostic histologic features. In one such study by Shim et al.127 that involved assessing the effect of stromal lymphocytic infiltration in thymic carcinoma, patients with low levels of CD4+ lymphocytes and CD20+ lymphocytes within the tumor stroma were found to have lower survival rates, and various combinations of CD4+, CD8+, and CD20+ cells were particularly predictive of worse survival outcomes. These authors concluded that these lymphocytes may work together to suppress cancer progression, and that combinations of them may be useful for stratifying patients in terms of long-term prognosis.127
Molecular Characterization
During the past several years, pathologic classification systems for several thoracic malignancies have been improved by molecular characterization of tumors, and this innovation is relevant in thymomas as well. Several studies have shown elevated expression of the epidermal growth factor receptor (EGFR) in large percentages of thymic malignancies, as is also true for non–small cell lung cancer and other epithelial cancers.128,129–131 However, EGFR mutations are rare in thymic malignancies,132,133 and as a result, targeted EGFR inhibitors have not shown consistent improvements in thymic tumor response or control rates.134,135–136 Other signaling pathways that often show overexpression are the VEGF,137 insulin-like growth factor-1R (IGF-1R),138 and KIT pathways,139,140 although in most circumstances the overexpression takes place mainly in thymic carcinomas rather than thymomas. Phase I and II trials with agents targeting receptors in these pathways, like those with EGFR-targeted agents, have been largely disappointing,141,142 although figitumumab, an anti-IGF-1R antibody, has shown efficacy in early clinical trials for advanced or refractory thymomas,143,144 and phase II studies are ongoing. In addition, a phase II study evaluating the efficacy of the histone deacetylase inhibitor belinostat in patients with recurrent or refractory advanced thymic epithelial tumors showed that of 41 patients enrolled (25 with thymoma, 16 with thymic carcinoma), the response rate was 8%, with median times to progression and survival of 5.8 and 19.1 months, respectively. These investigators concluded that this agent had “modest” antitumor activity in this setting, but the prolonged duration of the response warranted further study.145
Although steroid receptor expression has been thoroughly studied in other malignancies such as breast cancer, less research on this topic has been done in thymic malignancies. Glucocorticoid receptors are known to be expressed in epithelial cells, and the delivery of glucocorticoids can induce apoptosis in thymocytes.146,147 Indeed, prednisone has been shown to produce significant responses in patients who had not responded to other agents. A recent study evaluating the presence of glucocorticoid, estrogen, and progesterone receptors in thymomas and thymic carcinomas showed that glucocorticoid and estrogen receptors were overexpressed in 83% and 76% of thymic malignancies, and progesterone receptors were overexpressed in <1%. Glucocorticoid receptor expression was also associated with better prognosis among patients who underwent resection.148 Studies such as these may lead to the discovery of novel, less-toxic therapies for patients with primary, metastatic, or refractory disease.
Staging
The most commonly used staging system for thymomas was published by Masaoka et al.103 in 1981 (see Table 52.3). Staging is based on the extent of either macroscopic or microscopic invasion into mediastinal structures at the time of surgery. Other groups have tried to improve upon the Masaoka system. For example, the French Groupe d’Etudes des Tumeurs Thymiques classification (Table 52.4) incorporates completeness of resection, which may be of prognostic value but does not allow patients to be compared independent of treatment factors.149
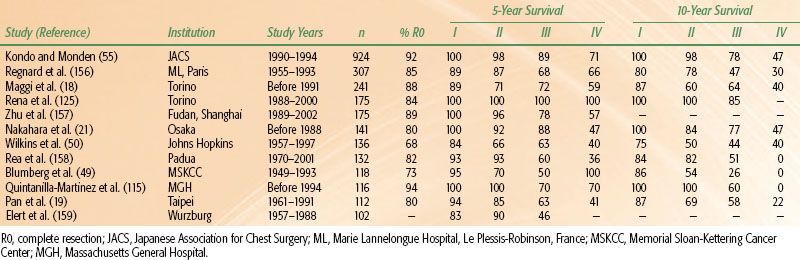
TABLE 52.5 OVERALL SURVIVAL RATES FOR PATIENTS WITH THYMOMAS AT 5 AND 10 YEARS
Prognostic Factors
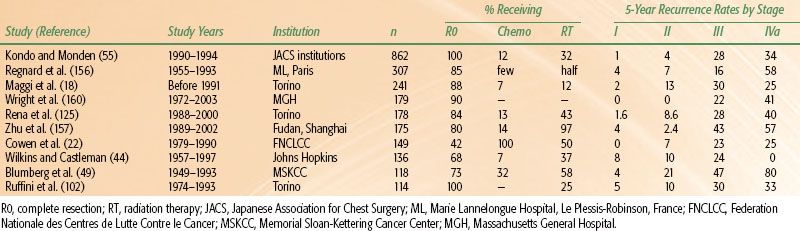
The two factors that have consistently demonstrated prognostic value in multivariate analyses in large studies are tumor invasiveness (i.e., disease stage) and completeness of resection.16,17,19,20,34–36,41,49,50,74,100,103,150,151–154 Disease stage has proven important for prognosis in every large study.16,17–18,20,21,42,49,50,100,103,155 Mean overall survival rates at 15 years are 78% for those with stage I disease, 73% stage II, 30% stage III, and 8% for stage IV (Table 52.5).17 At 10 years, mean disease-free survival rates are 92% for those with stage I disease, 87% stage II, 60% stage III, and 35% for stage IV (recurrence rates are summarized in Table 52.6).17,20,100,115,154
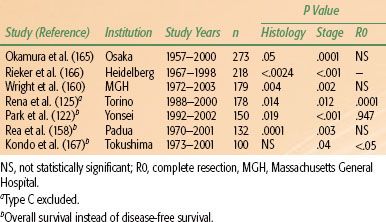
The extent of resection is the other major factor consistently identified as being prognostic in thymic malignancies.17,49,50,161–163 Patients for whom complete (R0) resections can be done have significantly better survival than do those with R1 or R2 resections.103,164 R0 resections are almost always possible for stage I tumors, but resectability rates decrease on average to 50% for stage III tumors.18,21,42,103 The WHO histology classification also was recently found to be independently associated with prognosis. Major studies reporting analyses of stage, histology, and resection status as independent predictors of survival in thymic tumors are summarized in Table 52.7.
Other potential prognostic factors are tumor size (>10 cm) and the presence of symptoms.16,49,105 In older series, patients with parathymic syndromes such as myasthenia gravis and other autoimmune diseases fared worse than those without such syndromes,44,49,154 but this finding has not been replicated in newer series.17,20,21,50,75,76,103,105,152,168–170 Indeed, some studies have found survival to be better for patients with myasthenia gravis,18,50,171 perhaps because of earlier detection of thymomas.49,172–173,174 A study published in 2005 indicated that myasthenia gravis, present in 25% of 1,089 patients from Japan,56 did not affect 5-year overall survival rates among patients with stage III disease. For patients with stage IV disease, 5-year overall survival rates were 85.1% for patients with myasthenia gravis versus 63.9% for patients without. R0 resection was accomplished in a significantly higher proportion of patients with myasthenia gravis (60%) than those without it (38%).
Another potential prognostic factor is patient age. Patients older than 30 to 40 years may have a better prognosis than younger patients.16,74,154 Thymomas in children seem to follow a more malignant course than those in adults.175 Fortunately, malignant thymomas in children are extremely rare.176
TABLE 52.6 RECURRENCE RATES OF THYMOMAS
General Management
Surgery
Surgical resection is the mainstay of treatment for thymomas. A complete en-bloc surgical resection (R0) remains the treatment of choice for all thymomas regardless of invasiveness, except in rare advanced cases with extensive intrathoracic or extrathoracic metastasis. Fortunately, the vast majority (90% to 95%) of thymomas are localized.177 Operative mortality rates average 2.5% (range, 0.7% to 4.9%).16,17–18,21,42,49,96,159 Resectability rates for stage I thymomas should approximate 100%, but those rates vary widely for higher-stage thymomas: 43% to 100% (mean, 85%) for stage II, 0% to 89% (mean, 47%) for stage III, and 0% to 78% (mean, 26%) for stage IV.100
Because the completeness of resection is such an important prognostic factor, an aggressive surgical approach is justified to remove as much of the lesion as possible at surgery. If residual microscopic disease is suspected of being present (an R1 operation), metallic clips should be placed to help delineate the radiation field. Whether subtotal resections are beneficial is controversial, with some authors demonstrating better survival when debulking takes place before adjuvant radiation therapy18,21,55,103,104,154 and others finding no benefit from debulking over biopsy alone.17,18,21,49,74,149,154,171,178 Another large study found significant differences in 5-year survival rates among patients undergoing subtotal resection versus biopsy only (64% vs. 36%) but little difference in 10-year survival rates, suggesting that any benefit may be only in intermediate-term survival.55 Because of the difficulties in controlling for selection bias and confounding influences of other treatments or disease-related variables, no consensus has been reached as to whether subtotal resections in general are beneficial or not. It is quite possible that a planned incomplete resection that leaves minimal residual disease will be advantageous, especially in the context of adjuvant radiation or chemotherapy.
Advances in surgical techniques for thymectomy have included the use of robotics. Potential advantages of robotic techniques include increased rates of preserved pulmonary function, improved cosmesis, and higher rates of patient compliance because of the minimally invasive nature of the surgery.179 Several institutions are exploring the use of robotics in thymectomy, and a few studies have been published. Rückert et al.180 demonstrated the feasibility of this technique for patients undergoing thymectomy for myasthenia gravis. That same group of investigators then published a retrospective cohort study comparing robotic and nonrobotic thoracoscopic thymectomy and found that rates of remission of myasthenia gravis were actually higher for patients who had received a robotic thymectomy; the authors attributed this finding to the potential for improved mediastinal dissection with the robotic technique.181
Another minimally invasive procedure is video-assisted thoracoscopic extended thymectomy (VATET). A group in Japan used this procedure to treat 35 patients with clinical stage I thymoma from 1998 to 2009.182 The authors reported the technique to be safe, with no perioperative deaths and three minor complications. Disease in 20 of the 35 patients was upstaged to Masaoka stage II or III at the time of surgery. At an average follow-up time of 65 months, one patient had experienced recurrence in the bilateral lung.182 Future studies will need to examine the efficacy and safety of both robotic surgery and VATET for thymic malignancies.
Traditional surgical techniques for patients with stage I thymic tumors produce 5-year survival rates in excess of 90%, with survival rates decreasing slightly at 10 years18,21,64 and with local recurrence rates of <5%. For stage II and III disease, recurrence rates after surgery alone range from 10% to 47%. In a study of more than 100 patients previously treated for thymoma, causes of death were as follows: 38% were related to thymoma (range, 19% to 58%), 9% to postoperative causes (range, 2% to 19%), 22% to myasthenia gravis (range, 16% to 27%), 9% to other autoimmune diseases (range, 2% to 19%), and 29% to unrelated causes (including other cancers) (range, 8% to 47%).16,17–18,20,21,42,50,101,104,115,154 Recurrence rates are summarized in Table 52.6.
TABLE 52.7 RESULTS OF MULTIVARIATE ANALYSES OF FACTORS PREDICTING THYMOMA-SPECIFIC SURVIVAL
Patterns of Failure
The pattern of failure in the overwhelming majority of thymomas is locoregional: 81% of recurrences are local, 9% are distant, and 11% are both.16,17,42,49,101,102 Most recurrences arise within 3 to 7 years,16,17–18,49,50,102 but recurrence has been documented as late as 32 years after the initial resection.42,57,169,183,184 The treatment for recurrence is usually surgery and adjuvant radiation.73,102,185 Most recurrences (50% to 75%) are operable, and of those that are operable, the reported rates of a successful R0 resection range from 45% to 71%.49,73,100,102,186 Patients with a recurrence after an R0 resection generally experience acceptable short-term and long-term results,73,185 with 10-year actuarial survival rates ranging from 53% to 72%73,100,102,187; 10-year survival rates after an incomplete resection, by contrast, range from 0% to 35%.17,73,100,102,187 In one recent study from Memorial Sloan-Kettering Cancer Center, of 25 patients who experienced recurrent disease after initial resection, 11 (44%) had disease that was amenable to re-resection. In that same study, 50% of patients undergoing surgery for recurrent disease had an R0 resection, but 82% of these patients experienced a second recurrence. The authors concluded that “despite the historical enthusiasm for re-resection … reoperation should be considered only in selected patients.”188
When recurrent disease is unresectable, radiation and chemotherapy have been used with modest results: 5-year overall survival rates reportedly range from 25% to 50%, with poorer longer-term survival.18,49,100,102,186,189
Radiation Therapy
Adjuvant Radiation After Complete Resection
Radiation therapy can be considered as an adjuvant treatment for patients with resected stage II and III thymomas, although recurrence rates for stage I thymomas after an R0 resection are so low that radiation is considered unlikely to offer improvement. The indications for radiation are controversial, with some recommending adjuvant radiation for all patients,21,104 others recommending adjuvant radiation for stage II and III thymomas,17,64,77,149,154 and still others recommending radiation only after an incomplete resection.55,149,154 All of the reported findings regarding adjuvant radiation are retrospective and, because of the rarity of thymoma, they span many decades. Because disease stage and completeness of resection are such important prognostic factors, any analysis of adjuvant radiation must be considered in light of these factors.
Several studies have shown trends toward better local control after adjuvant radiation for completely resected stage II thymoma; one of the largest studies found no difference in recurrence rates,55 and another series reported worse results with adjuvant radiation.102 Haniuda et al.190 found that patients with fibrous adhesion to the mediastinal pleura without microscopic invasion benefited the most from postoperative therapy: recurrence rates among patients with such adhesion were 36.4% versus 0% among those without adhesion. Thus, mediastinal pleura invasion may be another factor to consider. Another study by Chang et al.191 of patients with completely resected stage II or III thymoma demonstrated similar overall survival rates but improved disease-free survival rates with adjuvant radiation therapy (93% with radiation vs. 70% without). However, several other studies have shown that radiation after resection of stage II thymomas is not beneficial. In one such study, Berman et al.192 found that the local recurrence rate among 74 patients after complete resection for stage II disease was only 3% and was not affected by receipt of adjuvant radiation. Utsumi et al.193 reported similar findings in a study of patients with stage I or II completely resected disease. Finally, a SEER analysis of 901 patients showed no clear benefit from adjuvant radiation for patients with completely resected disease.194
Unlike these studies, many others (most with fewer than 50 patients) have reported that adjuvant radiation, after R0 resection for stage III thymoma, produces high rates of local control. Urgesi et al.,152 in a study of 33 patients, reported no in-field recurrences and only 3 out-of-field recurrences, but others have found that adding radiation therapy did not affect local or distant recurrence rates.49,55,195–197 On the other hand, some studies of completely resected stage II and III thymomas have demonstrated marginal benefits from the use of postoperative treatment.18,37 The authors recommend that the choice of whether to use adjuvant treatment for stage II or III disease should be made on an individual basis and should consider risk factors such as frank pleural or pericardial invasion, tumor grade, and comorbid conditions. The authors further strongly encourage that proposed treatment strategies be discussed in a multidisciplinary setting before the final recommendation is made.
Adjuvant Radiation after Incomplete Resection
Radiation is often considered when complete resection is not possible. Two studies have suggested that adjuvant radiation can be beneficial for patients with subtotally resected thymomas.18,37 Unfortunately both studies were small and, as always, subject to selection bias. Another study of 44 patients who had had R0 or R1,2 resections of stage III thymoma showed that adjuvant radiation produced lower recurrence rates (40% vs. 24% without radiation) and may have reduced the recurrence rates among patients with stage IV disease as well.37 Curran et al.37 reported no mediastinal failures after radiation in 26 patients with incompletely resected stage III thymoma compared with 79% at 5 years among patients who did not receive radiation. Other investigators have concurred, reporting very low rates of mediastinal failure among patients with gross residual disease treated with adjuvant radiation.152,198
Radiation as Neoadjuvant Therapy
Radiation has been proposed as a neoadjuvant strategy to reduce tumor burden and improve resectability, especially for cases involving gross invasion of critical structures.34,106,155,199–203 Response rates of up to 80% have been reported, and a theoretical decrease in the potential for tumor seeding during surgery has been proposed as well.18,37,154,199 The rates of R0 resections after neoadjuvant radiation for stage III thymoma can be as high as 53% to 75%,106,203 which are favorable compared with the typical 50% rate of R0 resections of stage III thymomas.17,20,21,37,49,77,103,149 Ten-year survival rates do not seem to be better after preoperative radiation, but to date the studies evaluating this approach have been small.106,202,203
Radiation as Definitive Therapy
Radiation therapy alone has been used for patients who cannot undergo surgery because of medical conditions or those for whom surgical resection is not possible, with modest results. Arakawa et al.204 reported that 7 of 12 patients presenting with unresectable tumors treated with primary radiation therapy were still alive at follow-up times ranging from 1 to 5 years. Ciernik et al.198 reported a 5-year survival rate of 87% for a small group of patients with stage III and IV disease who underwent radiation without resection, and Jackson and Ball205 found 10-year survival rates of 44% for patients who received radiation after biopsies or incomplete resections. As for the use of radiation for recurrent disease, Urgesi et al.186 reported outcomes of 21 patients given radiation alone after intrathoracic recurrences of thymoma. The 7-year survival rate of 70% was similar for those treated with radiation alone as for those treated with surgery and adjuvant therapy. Although these results are informative, in the era of combined-modality therapy, the use of preoperative and definitive radiation therapy without chemotherapy is no longer a primary consideration in the management of difficult thymomas.
Chemotherapy
Thymomas are quite sensitive to chemotherapy, with approximately two-thirds of patients showing a clinical response and one-third experiencing a complete response.67,170,206–211,212–213 The duration of response ranges from 12 to 93 months. Whether chemotherapy influences long-term survival is more difficult to assess. In one retrospective analysis of 90 patients, chemotherapy reduced the rates of metastases to the lung, pleura, or other sites by half (17% vs. 38%; P <.05). All of those patients had stage III or IV tumors and were treated with radiation and partial or no resection.154 Another study reported a nonsignificant trend toward better disease-free survival from the addition of chemotherapy.178
The most promising use of chemotherapy is in the neoadjuvant setting. Like preoperative radiation, chemotherapy seems to render tumors more suitable for complete resection. One study demonstrated that neoadjuvant chemotherapy was associated with improved survival for patients with stage III or IVa thymomas.174
As is true for the literature on the effects of radiation, most of the series describing the use of chemotherapy are small and retrospective. Drugs commonly used in combination chemotherapy include cisplatin, doxorubicin, and cyclophosphamide. One prospective intergroup study reported disappointing results with combined etoposide, ifosfamide, and cisplatin.207 A more recent study of patients with advanced disease showed that the combination of carboplatin and paclitaxel produced response rates of 43%, with a median survival time of 20 months. The authors of that report concluded that the clinical activity of that combination was less than that of anthracycline-based therapy.214
Aside from cytotoxic agents, somatostatin analogs (e.g., octreotide) and high-dose corticosteroids have shown promise in thymomas.215,216 In one prospective study, two courses of glucocorticoid therapy before surgery led to a 47% response rate among 17 patients with resectable thymomas.217 This therapy seems to work by exploiting the ability of corticosteroids to induce apoptosis in CD4+CD8+ immature thymocytes. Another prospective study by the Eastern Cooperative Oncology Group enrolled 42 patients with unresectable, advanced thymic malignancies for whom octreotide scans were positive. Patients were treated with octreotide with or without prednisone. Two patients had complete responses and 10 had partial responses, which led the investigators to conclude that octreotide alone had modest activity and prednisone improved the overall response rate.218 More recent molecular-level studies of signaling pathway activation have implicated c-KIT and EGFR in thymic malignancies,142 and some isolated observations of response to targeted therapy such as cetuximab have been noted, but the therapeutic implications of these observations remain to be determined.
Combined Modality Therapy
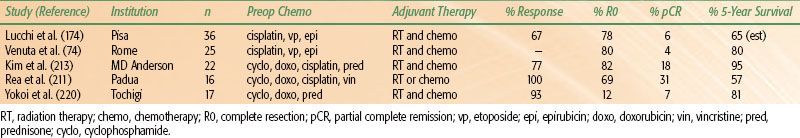
Some evidence exists to suggest that multimodality treatment can improve resectability and survival among patients with stage III or IV thymomas; typical combinations include neoadjuvant chemotherapy followed by surgery and postoperative radiation, chemotherapy, or both. Prospective trials of preoperative combination chemotherapy have been undertaken at several institutions74,211,213,219; the regimens in all cases included cisplatin with some combination of cyclophosphamide, doxorubicin, vincristine, prednisone, or epirubicin. Reported response rates to these regimens range from 77% to 100% (Table 52.8); R0 resections were possible in 57% to 82% of cases; and pathologic complete response rates ranged from 4% to 31%. Overall survival rates at 5 years (57% to 95%) were quite favorable for unresectable stage III or IV thymoma.74,211,213,219,221 In many of these studies, most if not all patients received postoperative radiation (see Table 52.8), and the results seem superior to historical results from patients who underwent surgical resection alone. In summary, multimodality therapy consisting of preoperative combination cisplatin-based chemotherapy followed by surgery and postoperative radiation can produce excellent results.
TABLE 52.8 OUTCOMES AFTER COMBINED MODALITY THERAPY FOR THYMOMAS