Thomas W. Geisbert
Marburg and Ebola Hemorrhagic Fevers (Filoviruses)
Viral hemorrhagic fever (VHF) is a syndrome characterized by fever, malaise, myalgia, and blood coagulation disorders that can progress to multiorgan failure, shock, and death in many cases. VHF is caused by members of four different families of RNA viruses. Among the VHF members of the family Filoviridae, Marburg virus (MARV) and Ebola virus (EBOV) are the most feared because of their dramatic clinical presentation, unusually high case-fatality rates of up to 90%, and because their natural history remains a mystery. In addition to concerns of natural outbreaks in regions of Central Africa, EBOV and MARV are known to have been the subjects of former biological weapons programs and have the potential for deliberate misuse (see Chapter 15).1,2 Currently, there are no filovirus vaccines or treatments approved for human use. For these reasons, EBOV and MARV have recently been included as only 2 of 11 human pathogens and only 2 of 4 viruses on the new United States Department of Health and Human Services Tier 1 list of Category A select agents (the other two viruses are variola major and minor).3 In addition to causing significant disease in humans, filoviruses have decimated populations of great apes in the Congo basin, further impacting an already endangered species.
Virus Characterization
The family Filoviridae is divided into two genera: MARV and EBOV. Although the MARV genus contains a single species, the EBOV genus consists of five distinct species: Bundibugyo ebolavirus (BEBOV), Côte d’Ivoire ebolavirus (CIEBOV; also known as Ivory Coast ebolavirus), Reston ebolavirus (REBOV), Sudan ebolavirus (SEBOV), and Zaire ebolavirus (ZEBOV).4 Nucleotide and amino-acid differences between MARV and EBOV are each approximately 55%, and there is no serologic cross-reactivity between these viruses. In comparison, EBOV species show 37% to 41% differences in nucleotide and amino-acid sequences, and there are varying degrees of cross-reactivity among the EBOV species.
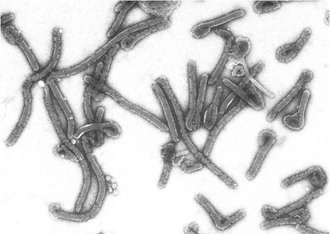
Filoviruses are enveloped, nonsegmented, negative-strand RNA viruses. Filovirus particles take on a variety of forms, from circular or “6”-shaped to prototypical straight filaments, for which the virus family is named (Fig. 166-1). Although the length of the virions is variable, MARV particles average close to 800 nm, and EBOV virions measure about 1 µM. The diameter of all filovirus particles uniformly measures about 80 nm.5 Filovirus particles contain an approximately 19-kb noninfectious genome that encodes seven structural proteins, with a gene order of 3′ leader, nucleoprotein (NP), virion protein 35 (VP35), VP40, glycoprotein (GP), VP30, VP24, RNA-dependent RNA polymerase L protein, and 5′ trailer. Four of these proteins are associated with the viral genomic RNA in the ribonucleoprotein complex: NP, VP30, VP35, and the L protein. Some proteins of the ribonucleoprotein complex have additional functions. For example, VP35 has been shown to act as an interferon antagonist.6 VP40 serves as the matrix protein and mediates particle formation, and in the case of MARV, it has also been shown to interfere with host innate immune responses.7 VP24 is another structural protein associated with the membrane and also interferes with interferon signaling for EBOV.8
The GP is the surface glycoprotein that forms the spikes on the virion and is the effector for receptor binding and membrane fusion. An important distinction of EBOV from MARV is that the MARV GP is encoded in a single open reading frame (ORF), whereas the EBOV GP is encoded in two ORFs.9,10 The single MARV ORF translates into the structural surface GP. In contrast, the two EBOV ORFs are linked together by slippage of the L polymerase at an editing site (a string of seven consecutive template uracil residues) to insert an eighth uracil. This process results in the production of a messenger RNA (mRNA) transcript that permits read-through translation of full-length GP. However, only about 20% of the mRNA transcripts are edited and translated into structural surface GP. The remaining 80% of unedited mRNA transcripts result in the production of a truncated soluble GP (sGP) that is secreted in large quantities from infected cells. Although the function of sGP has not been fully elucidated, it has been postulated that sGP subverts the host immune response by both passively absorbing antibodies directed at the full-length structural GP11,12 and by triggering the proliferation of B cells that preferentially bind sGP.13
Epidemiology
Marburg Hemorrhagic Fever
The first documented outbreak of VHF caused by a filovirus occurred in 1967 when there were three concurrent episodes of lethal MARV infections in Marburg and Frankfurt, Germany and in Belgrade (in the former Yugoslavia) among laboratory workers exposed to blood and tissue products of African green monkeys imported from Uganda (Fig. 166-2).14 Secondary transmission to medical staff and family members was also documented. In total, 31 patients became infected, and 7 of these patients died. During the next 2 decades, MARV was associated with sporadic, isolated, usually fatal cases among residents and travelers in southeast Africa.
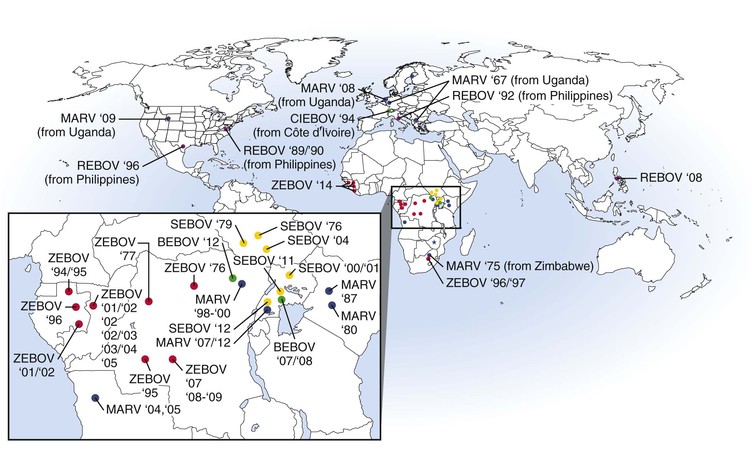
In 1998 to 2000, there was a prolonged outbreak involving 154 cases of MARV hemorrhagic fever (HF) in Durba, Democratic Republic of the Congo (DRC) that was associated with individuals working in an underground gold mine.15 Case-fatality rates from this outbreak are unclear but may be up to 83%. This outbreak was unique and complicated by the fact that it had multiple introductions of MARVs of different phylogenetic lineages and included strains that are thought to be more pathogenic (Angola)16,17 than others. The largest and most lethal MARV outbreak to date occurred in 2004 to 2005 in northern Angola.16 This outbreak involved 252 cases, with a case-fatality rate of 90%. The epidemic was driven largely by nosocomial transmission; however, community-acquired infection was documented toward the end of the outbreak. Between 2007 and 2012, several small episodes of MARV HF were reported in Uganda, with one case being exported to the United States18 and one to the Netherlands.19
Ebola Hemorrhagic Fever
EBOV was first recognized during near-simultaneous explosive outbreaks in 1976 in small communities in the former Zaire (now the DRC) and Sudan (see Fig. 166-2).20,21 There was significant secondary transmission through the reuse of unsterilized needles and syringes and nosocomial contacts. These independent outbreaks involved serologically distinct species, ZEBOV and SEBOV. The ZEBOV outbreak consisted of 318 cases and 280 deaths (88% mortality), whereas the SEBOV outbreak involved 284 cases with 151 deaths (53% mortality). Since 1976, ZEBOV has appeared sporadically in Central Africa, causing several small- to midsize outbreaks between 1976 and 1979. In 1995, there was a large epidemic of ZEBOV HF involving 315 cases, with an 81% case-fatality rate, in Kikwit, a community in the DRC.22 Meanwhile, between 1994 and 1997, there were smaller outbreaks caused by ZEBOV in Gabon. Since 2000, there have been near-yearly occurrences of ZEBOV in Gabon, DRC, or the Republic of Congo. During 2014, ZEBOV outbreaks were reported for the first time in West Africa in the countries of Guinea, Liberia, and Sierra Leone. The Central Africa outbreaks of ZEBOV have also involved a catastrophic decline in populations of great apes.23,24 The largest EBOV outbreak on record involved 425 cases, with a 53% case-fatality rate.25 This outbreak occurred in 2000 to 2001 in Sudan and was caused by SEBOV. Smaller outbreaks of SEBOV have occurred in Sudan in 2004 and in Uganda in 2011 and 2012.
In 1989 to 1990, a third species of EBOV, REBOV, appeared in Reston, Virginia in association with an outbreak of VHF among cynomolgus macaques imported to the United States from the Philippine Islands.26 Hundreds of monkeys were infected (with high mortality) in this outbreak, but no human cases occurred. Four animal caretakers seroconverted to REBOV with no overt disease. Epizootics in cynomolgus monkeys recurred at other facilities in Europe and the United States through 1992 and again in 1996. Subsequently, REBOV has been found in the Philippines on several occasions, with surprising reports documenting infections in domestic pigs.27
A fourth species of EBOV, CIEBOV, was identified in Côte d’Ivoire in 1994.28 The virus was isolated from an ethnologist who had worked in the Tai Forest reserve and became infected after a necropsy on a chimpanzee. The individual became ill with symptoms consistent with filovirus infection and survived infection. The chimpanzee originated from a troop that lost several members to an illness that was subsequently identified as being caused by CIEBOV.
The latest and fifth species of EBOV, BEBOV, was discovered in Uganda late in 2007 during an outbreak that involved 56 confirmed cases and an approximate 40% case-fatality rate.29 A more recent outbreak of BEBOV occurred late in 2012 in the DRC and involved 52 probable cases and a 48% case-fatality rate.30
Natural History
Human and nonhuman primates are susceptible to filovirus infection and are considered to be end hosts rather than potential reservoirs. Surveys to identify animal reservoirs and arthropod vectors have been aggressively undertaken in endemic areas, particularly after most large filovirus outbreaks. Until recently, these efforts have been unsuccessful. Ecologic studies in 2003 to 2006 in Gabon and the Republic of Congo demonstrated the initial evidence for the presence of ZEBOV in three different species of fruit bats.31 These studies showed the presence of viral RNA and antibodies, although the investigators were unable to isolate infectious ZEBOV. Subsequent studies in 2007, detecting MARV RNA and isolating infectious MARV from cave-dwelling fruit bats in Uganda, further support the view that bats may serve as a reservoir for filoviruses.32 More recently, antibodies against REBOV were detected in fruit bats in the Philippines.33 Although current data suggests a role for bats in maintaining filoviruses in nature, it remains unclear whether bats serve as the primary reservoir or whether other species are involved.
Clinical Manifestions and Diagnosis
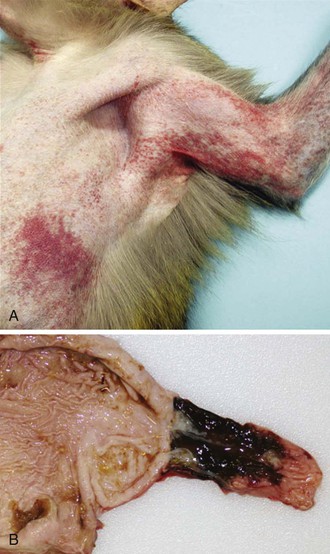
Clinical and laboratory features of MARV and EBOV infection are nonspecific and include an incubation period of 2 to 21 days (mean, 4 to 10 days) with a sudden onset of fever, malaise and/or myalgia, and may include a variety of other nonspecific symptoms.4,34 The presence of an erythematous, maculopapular rash may be observed (Fig. 166-3). A constellation of other coagulation disorders may occur, including bleeding from venipuncture sites and the gastrointestinal tract (see Fig. 166-3). Clinical pathology findings include leukopenia and lymphocytopenia with increased levels of neutrophils, thrombocytopenia, and increased serum levels of the liver-associated enzymes aspartate aminotransferase and alanine aminotransferase. Prolonged blood coagulation times and increased circulating levels of D-dimers are also associated with filovirus infections.35,36