Mantle Cell Lymphoma
Mantle cell lymphoma is neoplasm derived from B cells that normally occupy the mantle zone surrounding the lymphoid follicle. Mantle cell lymphomas account for about 8% of non-Hodgkin lymphomas. They contain a characteristic cytogenetic abnormality, t(11;14)(q13;q32), that results in the overexpression of cyclin D1 (1).
CLINICAL FEATURES
Patients with mantle cell lymphoma are typically older white men. The disease usually (90%) presents in advanced stage (III or IV) and very often includes extranodal involvement of the bone marrow (80%), gastrointestinal tract (80% histologically involved, 25% symptomatic), and liver, in addition to lymphadenopathy and enlarged spleen and nodal tissue in Waldeyer’s ring. The peripheral blood may show lymphocytosis but clonal abnormal cells are detectable by flow cytometry even when lymphocytosis is absent. B symptoms are present in 20%. Cells overexpressing cyclin D1 are detected in asymptomatic normal people rarely (5%–8%), but it is not clear that their presence presages the development of lymphoma. Involvement of the gastrointestinal tract may produce multiple lymphomatous polyposis.
The natural history of the disease is variable. About 15% of patients may have an indolent clinical course. In the extreme, a patient may not manifest progressive disease over many years of follow-up without treatment. However, the majority of patients show disease progression within the first 1 or 2 months of diagnosis. About 10% of patients show extremely rapid disease progression and follow a downhill course in a few months. The usual patient responds to chemotherapy but complete remissions are seldom durable and the median survival is 3–5 years (2).
PATHOLOGY AND GENETIC FEATURES
Mantle cell lymphomas are not graded according the WHO Classification of Lymphoid Tumors but classical variants and highly aggressive variants (blastoid and pleomorphic) are recognized. The classical form involves vaguely nodular sheets of small to intermediate size lymphoid cells often with a notched nuclear contour. The overexpression of cyclin D1 is a hallmark and is usually associated with the characteristic t(11;14)(q13;q32) translocation juxtaposing the cyclin D1 gene with the immunoglobulin heavy chain gene. Rare mantle cell lymphomas do not overexpress cyclin D1; these tend to overexpress cyclin D2 or D3. An unusual translocation of cyclin D2 to the immunoglobulin kappa light-chain gene [t(2:12)(p12;p13)] has been observed in some cases. The pattern of gene expression in these unusual variants mimics the pattern seen in cyclin D1-overexpressing tumors (3).
The malignant cell of mantle cell lymphoma expresses surface immunoglobulin (IgM and IgD) intensely, CD20, and CD5, but not CD10 or BCL6. Cyclin D1 and BCL-2 are expressed in the cytoplasm and are thought to be key to tumorigenesis. Cyclin D1 expression interferes with the G1 checkpoint (controlled by RB, the retinoblastoma protein, p27kip) at which the cell takes inventory and assesses its ability to enter DNA synthesis. BCL-2 prevents the programmed cell death that would normally occur in cells that were not ready for the G1/S transition (4). The cells accumulate genetic damage through defects in DNA repair mechanisms and the molecular profile is marked by overexpression of proliferation-related genes (5). The immunoglobulin variable region genes of the tumor cells are germline in the majority of cases but an important subset of the tumors have hypermutated variable regions suggesting that antigen selection may play a role in some tumors. Tumor cells also express SOX11 but its role is unclear. SOX11 is generally not expressed in other forms of lymphoma.
Many studies also suggest a role for the Ki-67 index in predicting outcome. Ki-67 is expressed in all phases of the cell cycle but is not expressed in resting cells; thus, it is a marker for growth fraction. The index is the fraction of cells in a tumor expressing Ki-67; in general, the higher the index, the more aggressive the tumor. A difficulty in applying the index clinically is the absence of standardization and the absence of wide availability of the test. The study of prognostic factors has not shown that Ki-67 staining adds independent significance to the clinical prognostic factors (6).
STAGING WORKUP
Once an excisional tumor biopsy has documented the diagnosis (needle aspirates are not indicated and only serve to delay definitive diagnosis), essential staging procedures are history inquiring about B symptoms and gastrointestinal tract symptoms, physical examination with careful measurement and documentation of involved lymph nodes, complete blood count with flow cytometry to assess the presence of monoclonal B cells, serum chemistry profile with special attention to lactate dehydrogenase (LDH) levels, bone marrow biopsy with or without an aspirate, and CT scan of the chest, abdomen, and pelvis. The role of positron emission tomography is not established. Sigmoidoscopy/colonoscopy is not necessary in asymptomatic patients but many of these patients will have lower GI tract involvement. Although a large fraction of patients have bone marrow involvement, the disease rarely involves the central nervous system. Sampling of cerebrospinal fluid and central nervous system prophylaxis are not indicated.
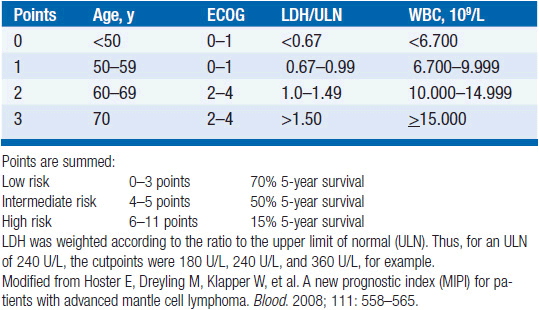
Patients can be grouped into low, intermediate, and high risk on the basis of four factors: age, performance status, LDH elevations, and white blood cell count (see Table 34-1). At the moment, therapy for mantle cell lymphoma is not risk-adapted; thus, the main value of the index is to predict outcome rather than guide decision making. An indolent disease subset can be identified by taking a watch-and-wait approach for a short time. Patients with indolent disease often have exclusively extranodal disease, tumor cells expressing hypermutated immunoglobulin variable region genes, and absence of aneuploidy. Most of these features are not readily available to the clinician, and thus the indolent subset is mainly recognized on the basis of absence of progression under clinical observation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree