Follicular Lymphoma
Follicular lymphoma (FL) represents 20%–30% of all non-Hodgkin lymphomas (NHL) and is the second most common NHL in Western populations after diffuse large B-cell lymphoma (DLBCL) (1). FL comprises about 80% of the indolent NHLs. The term follicular is derived from the tendency of the neoplastic cells to form microscopic nodules. The cell of origin is the follicular center B cell. Eighty-five to 90% of all cases harbor the characteristic cytogenetic translocation t(14;18), resulting in the placement of the anti-apoptotic bcl-2 gene under the control of the immunoglobulin (Ig) heavy-chain promoter on chromosome 14. Follicular lymphoma is considered incurable without stem-cell transplantation, with the exception of localized disease that may be cured with radiotherapy in a subset of patients. Treatment is therefore based on disease control rather than cure, and eventual relapse after treatment is the usual natural history of FL (2).
INCIDENCE
The incidence of follicular lymphoma is approximately 2.2–3.2 per 100,000 in the United States and Western Europe (3). Follicular lymphoma is more common in Caucasians than in people of African or Asian descent and typically affects the middle-aged or elderly with an average age at diagnosis of 60 years. The disease occasionally occurs in children or young adults. FL in children is a distinct clinical entity, presenting with localized disease that is often eradicated with initial therapy. In contrast, adults with FL typically present with advanced stage disease and experience recurrence after standard treatment. Although there are no universally accepted risk factors for the development of FL, herbicides and pesticides have been linked with the disease. Familial cases represent a small proportion of total incidence, and there appears to be a slightly increased risk in relatives of patients with FL.
PRESENTATION
Clinically, patients with FL often present with asymptomatic peripheral lymphadenopathy or have enlarged lymph nodes detected incidentally on imaging studies. Although hilar and mediastinal nodes are frequently involved, large mediastinal masses are uncommon. The spleen and bone marrow are commonly involved by disease, but CNS and organ involvement is uncommon. Infiltration of the bone marrow is present in 60%–80% of patients at time of diagnosis. The majority of patients (70%–80%) will present with advanced stage disease, and up to 25% will have B symptoms or an elevated serum LDH level. Although bone marrow involvement is common and sensitive molecular testing frequently identifies circulating lymphoma cells, cytopenias are uncommon at presentation.
In addition to the classic presentation of FL as described above, there are a few distinct clinical variants. Primary intestinal follicular lymphoma is often found incidentally on endoscopy performed for unrelated indications and most often involves the second portion of the duodenum. Pediatric lymphoma is another variant and has distinct features as noted above. Intrafollicular neoplasia, or in situ FL, refers to follicles with high levels of BCL-2 expression but without other features of FL. This condition has relatively low rates of progression to disseminated FL. Rarely, patients will present with diffuse large B-cell lymphoma (DLBCL) with concurrent, previously undiagnosed FL. Prognosis for these patients with transformation is significantly worse than for de novo DLBCL without underlying FL. Of note, DLBCL with t(14;18) translocation does not necessarily imply transformed FL. Histologic features of coexisting FL, most commonly presenting with involvement of the bone marrow with FL, in addition to DLBCL are required for the diagnosis of transformed FL. Histologic transformation most commonly occurs after a variable period of FL, is a natural feature of the disease rather than a side effect of treatment, and when it occurs, it signals an acceleration in the natural history of disease.
DIAGNOSIS
The diagnosis of FL is typically determined by examination of an involved lymph node, ideally by means of an excisional biopsy of a complete node. Fine needle aspirates are not adequate to fully assess the architecture of the disease and establish the grade of the lymphoma. Bone marrow biopsy is an important component of staging but does not allow for disease grading. FL is one of the few lymphomas where morphologic evaluation alone is often sufficient for diagnosis. Distinguishing FL from reactive follicular hyperplasia or other types of lymphomas may be difficult on occasion. FL most commonly appears as tightly packed nodules of varying size and shape consisting of a mixture of small lymphocytes with cleaved nuclei and large lymphocytes with noncleaved nuclei that efface the normal lymphoid architecture. This is in contrast to a normal lymph node, where follicles are more uniform in appearance and interfollicular elements are more prominent. Additionally, FL nodules typically have lower Ki-67 fractions and decreased numbers of phagocytic cells compared with reactive nodes. FL less commonly involves lymph nodes in a diffuse pattern and involved lymph nodes may have both nodular and diffuse areas of disease involvement. Bone marrow involvement typically appears as paratrabecular lymphoid aggregates.
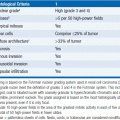
FL grading is based on the relative frequency of large noncleaved cells in the histologic specimen. Of note, grading of FL is notoriously nonconcordant between pathologists and with sequential readings by the same pathologist. Grading criteria are:
• Grade 1: 0–5 large cells per high power field (follicular small cleaved)
• Grade 2: 6–15 large cells per high power field (follicular mixed)
• Grade 3: >15 large cells per high power field (follicular large cell)
• 3A: small cleaved cells present
• 3B: small cleaved cells absent, solid sheets of large cells present
Grades 1, 2, and 3A are felt to represent a spectrum of disease, whereas the biology of grade 3B is distinct from the other subsets and cells typically lack CD10 and BCL-2 expression. Immunohistochemistry and flow cytometry demonstrate CD19, CD20, CD21, and CD79 positivity. CD10 is positive in up to 90% of cases. CD5, CD43, and CD11c are negative, and CD23 expression is variable although more commonly negative. The neo-plastic cells typically express monoclonal surface immunoglobulin, most commonly IgM or IgG. Cytoplasmic BCL-2 is strongly expressed in most grade 1, 2, and 3A FL, but is less commonly found in grade 3B disease.
Cytogenetic analysis demonstrates a BCL-2 translocation in 85%–90% of cases, typically as the result of a (14;18) translocation between the BCL-2 gene on chromosome 18 and the Ig heavy chain on chromosome 14. This translocation can also be found in up to 30% of cases of de novo DLBCL and occasionally in germinal center B cells in healthy individuals. Translocations between BCL-2 and the kappa light chain on chromosome 2 or lambda light chain on chromosome 22 are less common. All three translocations result in constitutive activity of BCL-2 leading to cellular resistance to apoptosis. BCL-2 translocations may also be identified by FISH and by PCR. The prognostic significance of minimal residual disease following therapy has not been clearly demonstrated. In addition, BCL-6 translocations on chromosome 3 are identifiable in 5%–15% of cases of FL and are more common in grade 3B disease, usually signifying a more aggressive clinical course. BCL-2 and BCL-6 translocations are not mutually exclusive, although FL harboring both mutations is uncommon.
STAGING AND PROGNOSIS
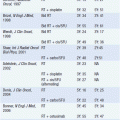
The Ann Arbor staging classification is employed in FL (4, 5):
• Stage 1: Limited to one lymph node region or lymphoid organ, or a single extranodal site (IE).
• Stage II: Limited to two or more lymph node regions on the same side of the diaphragm, or a single extranodal site with associated nodal involvement.
• Stage III: Involvement of lymph node regions or lymphoid organs on both sides of the diaphragm.
• Stage IV: Disseminated involvement of one or more extralymphatic sites, including liver, pleura, CNS, or bone marrow, with or without associated lymph node involvement.
Patients with persistent fevers, drenching night sweats, or a loss of >10% total body weight are considered to have B symptoms. Patients with FL are typically staged with computed tomography (CT) scans of the chest, abdomen, and pelvis as well as a bone marrow biopsy. Additional studies, such as CT scans of the neck, may be indicated based on individual clinical situations. FL is uniformly FDG avid on positron emission tomography (PET), and PET scans may upstage patients with FL compared with CT scan alone. Although a number of recent studies have examined the prognostic role of pretreatment, interim, and end-of-treatment PET scans, no clear guidelines exist on the use of this imaging modality and its use is currently investigational in FL (6, 7).
The best predictors of disease course and outcome are the factors that comprise the Follicular Lymphoma International Prognostic Index (FLIPI) score and disease grade. The FLIPI was developed using a retrospective multivariate analysis of over 4000 patients with follicular lymphoma from 1985 to 1992, before the routine use of rituximab. The prognostic value of the FLIPI has been validated in subsequent clinical trials (8, 9). The FLIPI score is based on the following criteria:
• Ann Arbor stage III of IV
• Hemoglobin <12.0 g/dL
• Involved nodal areas >4
• Serum LDH greater than the upper limit of normal
Five- and 10-year overall survival (OS) rates were determined based on the number of adverse factors present.
• Low risk (0–1 adverse factors): 91% and 71%
• Intermediate risk (2 adverse factors): 78% and 51%
• High risk (≥3 adverse factors): 52% and 36%
The FLIPI2 examined approximately 900 patients receiving therapy from 2003 to 2005 and identified the following criteria as independently prognostic (10):
• Age >60
• Bone marrow involvement
• Hemoglobin <12.0 g/dL
• Largest node >6 cm
• Elevated serum β2 microglobulin
The 3-year progression-free survival (PFS) was 91%, 69%, and 51%, and OS 99%, 96%, and 84% for patients with low (0), intermediate (1–2), and high (3) risk factors, respectively. Of note, only patients who received therapy were included in the analysis and the FLIPI2 prognostic score has not been prospectively validated in clinical trials.
Disease grade is also an important prognostic factor. Grades 1 and 2 disease have a similar clinical course and are treated uniformly. In general, grade 3 disease is more aggressive, though grade 3A disease may behave in a fashion more similar to grades 1 and 2 disease. Grade 3B is felt to represent a distinct entity and behaves more like DLBCL. Pathologic distinction between grades 3A and 3B disease may be difficult even with expert hematopathology consultation.
Finally, emerging data from gene expression profiling studies suggest that the tumor microenvironment and tumor immunology may predict disease behavior. Immune responses enriched with T cells as compared to those with higher percentages of monocytes/macrophages or dendritic cells appear to correlate with more favorable survival and lower rates of transformation to DLBCL (11, 12).
TREATMENT
Given that chemotherapy has not been curative in advanced stage FL and early therapy has not been shown to improve survival, the decision of when to begin treatment and the selection of therapy must balance symptoms from disease and influence of the cancer diagnosis on the patient with both disease- and treatment-related complications. The median survival for FL is in the range of 7–10 years, although extremes on either end occur. Overall survival may be significantly higher since the advent of rituximab. The 15%–20% of patients diagnosed with early-stage disease are potentially curable with local radiotherapy (RT). The mainstay of treatment for the remaining patients is systemic chemotherapy and/or immunotherapy, with a goal of disease control rather than cure. Radioimmunotherapy is another effective approach for some patients. More aggressive and toxic therapies may achieve better initial responses, but do not appear to translate into improved survival. As therapy has become more effective with incorporation of rituximab into combination chemotherapy regimens as well as its use as maintenance therapy, a larger fraction of patients achieve complete remissions and remissions are more durable than the median of 2 years achieved with older treatment regimens. For younger patients with relapsed or refractory disease, stem-cell transplantation may offer the potential for long-term control of the disease, at the expense of higher toxicity.
 TREATMENT OF EARLY STAGE DISEASE
TREATMENT OF EARLY STAGE DISEASE
Involved field radiotherapy (RT) may cure a subset of patients with nonbulky localized FL. RT leads to a 10-year OS of 60%–80% and median survival of 19 years, with some patients achieving cure (13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree