© Springer International Publishing AG 2018
Marissa Howard-McNatt (ed.)Changing Paradigms in the Management of Breast Cancer https://doi.org/10.1007/978-3-319-60336-0_44. Management of the Axilla in Breast Cancer
(1)
Department of Surgery, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA
Keywords
Breast cancerAxillaNode positiveAxillary lymph node dissectionSentinel lymph node surgeryAxillary ultrasoundACOSOG Z0011Neoadjuvant chemotherapyRegional nodal irradiationAxillary radiationIntroduction
In patients with breast cancer, axillary lymph node status is the most significant prognostic factor and predictor of long-term outcomes [1, 2]. Presence of nodal metastasis is associated with increased risk of locoregional recurrence and decreased overall survival. Patients who have nodal spread require more aggressive local and systemic therapy. Axillary lymph node dissection (ALND) was the standard of care for all patients presenting with breast cancer for much of the twentieth century for the purposes of staging and treatment. This procedure removes all the lymph nodes from level I and level II in the axilla and is associated with significant risk of functional disability of the ipsilateral arm, chronic pain, and lymphedema. It was not until the 1990s that a much less morbid procedure, sentinel lymph node (SLN ) surgery was developed [3, 4]. SLN surgery has been proven to be safe and feasible for clinically node-negative patients while accurately determining nodal status (node positive versus node negative). Management of the axilla has since evolved at an accelerated pace in recent years. Numerous multi-institutional studies have provided strong evidence that less aggressive surgical treatment of the axilla in most patients provide similar prognostic data while maintaining similar locoregional control.
Anatomy and Physiology
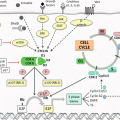
Lymphatic drainage of the breast originates from the breast lobules and flows into a subareolar plexus, that was first described by Sappey in 1874 who injected mercury into the dermis of a cadaver to identify the lymphatic pathways [5]. The axillary lymph nodes receive over 80% of the lymphatic drainage from all quadrants of the breast. The internal mammary, infraclavicular, and supraclavicular lymph nodes receive the remainder of the drainage.
The axillary nodes are divided into three levels described in relationship to the pectoralis minor muscle. Level I nodes are inferior and lateral, level II nodes are posterior, and level III nodes are medial to pectoralis minor. A complete axillary dissection involves removing level I and level II lymph nodes and any gross disease in level III.
SLN surgery for breast cancer was introduced in the early 1990s as an alternative to an axillary dissection for clinically node-negative patients for staging purposes. This concept utilizes the theory that the lymphatic drainage of the breast and any tumors that develop will first drain into one or a few lymph node(s) before spreading to the rest of the axilla. Thus, by locating and removing these lymph node(s), called the sentinel lymph node(s), the status of the axilla can be determined. In experienced surgeons, this procedure carries an overall SLN detection rate of 99% and has a false-negative rate (FNR ) between 5 and 10% [6].
The technique for SLN surgery involves injecting a tracer or two tracers into the breast to identify the SLNs. Most commonly used tracers are radioactive mapping agents and a blue dye (lymphazurin or methylene blue or patent blue dye). Numerous studies have shown that using dual agents increases SLN detection rates and minimizes SLN false-negative rates. The location of tracer injection was an area of debate in the past. Many surgeons initially injected the tracers around the tumor, with nonpalpable lesions being more difficult. However, several studies have shown that injecting in the subareolar space is easier, can be applied to multifocal tumors as well as nonpalpable tumors, and carries similar identification and accuracy rates to peritumoral injections [7, 8].
Clinically Node-Negative Patients
All patients initially diagnosed with breast cancer should undergo a complete history and physical. The physical should include an examination of the breast and axilla as well as the regional sites for lymphatic drainage, including the neck, supraclavicular, and infraclavicular regions. Unfortunately, physical examination alone is poor at assessing the axillary nodes as small metastatic lymph nodes may not be palpable and palpable lymph nodes may be reactive and not metastatic. Axillary ultrasound provides an extension of physical examination and provides a more reliable way to assess the axillary lymph nodes [9]. For this reason, an axillary ultrasound should be obtained and if any suspicious nodes are seen sonographically, a fine needle aspiration (FNA) biopsy or core needle biopsy should be performed. If the ultrasound-guided percutaneous biopsy does not show any metastatic involvement, patients are considered clinically node negative (cN0 ).
In the clinically node-negative patient with invasive breast cancer where surgery is the initial treatment, SLN surgery should routinely be performed to stage the axilla. Multiple randomized trials have proven that this is a safe and reliable technique, with equivalent overall and disease-free survival rates to ALND, but associated with much less morbidity.
The National Surgical Adjuvant Breast and Bowel Project (NSABP ) B-32 randomized 5611 patients across 80 institutions to SLN surgery plus ALND versus SLN surgery and completion ALND only in cases where any of the SLN(s) were positive [10]. The SLN identification rate was 97% overall with a false-negative rate of 9.7% in the SLN plus ALND group. B-32 did not show any differences in overall survival, disease-free survival, or recurrence rates between the two groups. This study validated the use of SLN surgery in clinically node-negative patients and led to SLN surgery replacing ALND as the standard procedure in staging the axilla in these patients.
When SLN surgery replaced ALND in clinically node-negative patients, sentinel nodes containing metastasis found at the time of surgery required an ALND. However, clinicians observed that in the majority of patients where a SLN was positive and an ALND was subsequently performed, there was no further disease in the additional axillary nodes resected in addition to the disease identified in the SLN. Clearing negative lymph nodes is not thought to provide oncologic benefit. This begged the question of whether patients with minimal axillary metastatic burden require ALND.
The American College of Surgeons Oncology Group (ACOSOG ) Z0011 trial by Dr. Armando Giuliano and colleagues challenged the dogma that patients with a positive SLN require an ALND [11, 12]. This trial randomized 891 patients who had a clinically negative axilla and were undergoing breast-conserving surgery for T1 or T2 tumors, planning adjuvant whole-breast radiation and were found to have only one or two positive sentinel lymph nodes into two groups: ALND versus no ALND. The majority of the patients received systemic adjuvant therapy. At a median follow-up time of 6.3 years, there were no significant differences in overall survival (91.8% versus 92.5%), disease-free survival (82.2% versus 83.9%), or axillary recurrence rates (0.9% versus 0.5%). More recently presented data provided longer follow-up results with a median of 9.25 years, showed that these results held true with longer follow-up. Nodal recurrence rates remained low and not significantly different between the two groups, 1.5% in the SLN group and 0.5% in the ALND group (p = 0.28) [13]. The authors concluded that in patients with early stage breast cancer with one or two positive sentinel lymph nodes undergoing breast-conserving surgery, SLN surgery alone is not inferior to ALND. The trial was criticized as the pre-specified accrual was not met, the majority of the patients had favorable tumor characteristics, and a significant number of patients were lost to follow-up in each group. In addition, the radiation fields were not documented and the degree of tangential radiation field coverage of the axilla or definitive radiation to the axilla is not known for the entire trial cohort. Radiation fields from a subgroup of 228 patients have been reviewed and showed that 19% of patients also had regional nodal irradiation , and this was more common in patients at higher risk for additional nodal involvement but, however, was not different between the two study arms [14]. Despite the criticism, the immediate impact Z0011 had on the national stage was irrefutable as surgeons have now adopted these findings into clinical practice and avoid ALND for patients meeting the Z0011 criteria.
The International Breast Cancer Study Group (IBCSG ) 23-01 randomized T1 or T2 breast cancer patients that were clinically node negative who underwent BCT or mastectomy and had micrometastases (defined as <2 mm) in the SLN to ALND versus no ALND [15]. There were 464 patients in the ALND arm and 467 patients in the no ALND arm. The authors found no statistical differences between the two groups in terms of 5-year overall survival (97.6% ALND versus 97.5% no ALND) and 5-year locoregional recurrence rates (2.4% ALND versus 2.8% no ALND). These findings corroborate the Z0011 findings, although 23-01 was limited to micrometastatic nodal disease. The proportion of mastectomy patients was too low to provide meaningful extrapolation to patients undergoing mastectomy.
Together, these recent trials have provided strong, randomized, multi-institutional data that SLN surgery alone in patients undergoing breast-conserving surgery found to have minimal disease burden in the axilla (micrometastases, one or two positive lymph nodes) is not inferior to a complete axillary dissection. Since ALND is associated with a greater morbidity and long-term complications compared to SLN, surgeons should omit ALND in patients with low-burden axillary disease undergoing breast-conserving therapy with adjuvant whole-breast radiation. However, an ALND should still be performed in patients who have three or more positive sentinel lymph nodes, or have fixed matted nodes, and in patients who are undergoing a mastectomy with any positive axillary lymph nodes.
The European trial EORTC 10981-22023 AMAROS (After Mapping of the Axilla, Radiotherapy or Surgery) was a multi-institutional trial randomizing patients with a positive SLN to ALND versus axillary radiotherapy [16]. There were 744 patients in the ALND group and 681 patients in the axillary radiotherapy group. In this trial, unlike in Z0011, mastectomy patients were allowed to participate. The authors found no statistical differences between the two groups in terms of 5-year overall survival (93.3% in the ALND group versus 92.5% in the radiation group) as well as 5-year disease-free survival (86.9% in the ALND group versus 82.7% in the radiation group), suggesting that radiation can be equivalent to ALND in early node-positive patients. However, many of these patients met Z0011 criteria and could potentially avoid both ALND and axillary radiation.
Clinically Node-Positive Patients
Axillary ultrasound at the time of diagnosis on all patients with invasive breast cancer helps provide preoperative staging information. Patients with suspicious-appearing lymph nodes on imaging, and FNA biopsy or core biopsy of an axillary lymph node proves metastatic disease are considered to be clinically node positive and this is designated with an (f) suffix [e.g., cN1(f)]. Placement of a clip in the node should be considered for cases where the biopsy shows metastatic involvement.
Historically, many patients who were operable candidates proceeded directly to surgery, while patients who were inoperable received neoadjuvant chemotherapy . In the modern era, there has been significantly greater use of neoadjuvant chemotherapy even in the setting of operable disease. While there is no evidence for a survival benefit with the use of neoadjuvant chemotherapy , this practice often allows for a reduction in the primary tumor burden in the breast and the extent of disease in the axilla as well as allowing assessment of tumor response. Patients who are not candidates for breast-conserving surgery often become candidates after neoadjuvant chemotherapy and patients are less likely to require ALND after neoadjuvant chemotherapy than if they undergo primary surgery.
The accuracy and false-negative rates of SLN surgery performed after neoadjuvant chemotherapy are similar to when SLN is performed upfront in clinically node-negative patients. The advantages of performing SLN surgery after neoadjuvant chemotherapy are as follows: (1) it allows the breast and axillary surgery to be performed at one operation rather than two separate operations, (2) less patients overall are node positive so fewer axillary dissections are needed, and most importantly (3) it allows assessment of response to therapy. If the SLNs are removed prior to chemotherapy, assessment of axillary response to chemotherapy cannot be performed.
For patients with clinically node-positive disease , historically ALND has been recommended after neoadjuvant chemotherapy. However, improvements in chemotherapy and targeted therapy have resulted in rates of nodal response to neoadjuvant chemotherapy as high as 40–75% [17–19]. Therefore, much recent interest has focused on the accuracy and feasibility of staging axillary response with SLN surgery following treatment, and reserving ALND for patients with residual nodal involvement rather than committing all patients to an ALND. Early studies demonstrated a higher than acceptable FNR of SLN surgery in this setting. In the last decade, several large, multi-institutional trials have been completed focusing specifically on assessing the accuracy of SLN surgery in node-positive patients treated with neoadjuvant chemotherapy .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree