Summary of Key Points
- •
Techniques for the optimal triage and preparation of small specimens for diagnosis and ancillary studies are provided.
- •
For optimal results, a standardized protocol and algorithm should be implemented in the laboratory.
- •
Various factors determine the decision to perform a core-needle biopsy or fine-needle aspiration, including operator and pathologist preference, availability of rapid onsite evaluation, risk of complications such as pneumothorax or hemorrhage, the possibility of tumor cell seeding, the type of lesion (epithelial or spindle cell), and the lesion’s location and size.
- •
Optimal use of a specimen requires appropriate handling and triage, with attention to both the quantity and quality of the specimen.
- •
Several measures can ensure that there is sufficient material in cores and cell blocks and that the tissue is not exhausted; the interventionalist should perform a gross examination of the specimen.
- •
In 2011, changes were made to the classification of adenocarcinoma to emphasize the interrelation of the tumor’s clinical, radiographic, and molecular characteristics.
- •
Cytologic preparations are important; each preparation is unique, and taken together, they offer complementary information that can be valuable in rendering a diagnosis.
- •
Smearing requires technical skill that is not always available or optimal.
- •
Cell blocks have been recommended instead of smears for use in ancillary tests by the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology (CAAP/IASLC/AMP) guidelines; yet despite the usefulness of cell blocks, there is no standardized procedure for processing them.
- •
Methods for the management of small biopsy samples in patients with lung cancer will improve in the future in order to maximize the use of material for diagnosis.
Lung cancer is the leading cause of cancer-related deaths worldwide, despite knowledge of the primary etiologic factor (tobacco use) and advances in identifying underlying mechanisms, detecting mutations, and developing new treatments. At one time, treatment selection depended on distinguishing small cell lung cancer (SCLC) from nonsmall cell lung cancer (NSCLC), but histologic subtyping of NSCLC has become increasingly important for selecting therapy. The addition of targeted therapies to the armamentarium for lung cancer necessitates testing for the presence of particular key driver mutations in lung adenocarcinomas to determine if a patient is eligible for a targeted therapy.
Small histologic and cytologic specimens obtained by core-needle biopsy and fine-needle aspiration are increasingly common. Use of computed tomography (CT), endobronchial ultrasound (EBUS), endoscopic ultrasound, and electromagnetic navigational bronchoscopy has enabled the collection of small specimens for more patients through minimally invasive procedures, replacing traditional methods for obtaining specimens, such as mediastinoscopy, video-assisted thoracoscopic surgery, and thoracotomy. Less invasive small specimen collection is especially useful for patients with advanced-stage cancer, nonsurgical diseases such as granuloma, or fibrosis after previous surgery, as well as for patients who are poor surgical candidates or who are undergoing restaging. In some cases, only small specimens are obtainable.
Small samples present a challenge: although the quantity of sample tissue being obtained is much smaller, the information required from the sample has increased substantially. In the past, little importance was placed on triaging small biopsy or cytologic specimens; however, now that treatment decisions are based on the histologic subtype and molecular profile of NSCLC, triaging the small specimens that comprise most or all of the diagnostic tissue has become crucial. A poorly managed specimen may preclude an accurate diagnosis. For patients with NSCLC, it is necessary to have sufficient tissue for ancillary studies, such as immunohistochemistry (IHC) and/or molecular tests, to determine treatment. Appropriate management is also necessary for cases that are not NSCLC.
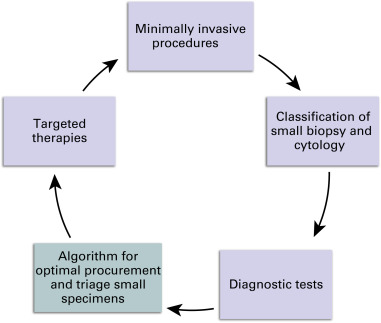
The shift to using small specimens for diagnosis has created a practice gap, because limited guidance is available for the management of small specimens ( Fig. 19.1 ). An optimal algorithm would define the steps for appropriate triage and processing of tissue to select a course of treatment. It would provide for a tissue reserve for identifying novel predictive biomarkers and suggest personalized therapies. Testing for mutations is likely to increase, and access to sufficient tissue will be crucial.
Specimens obtained by surgical biopsy are typically placed in formalin at the time of acquisition and then processed in the laboratory; this procedure rarely varies, and formalin fixation and hematoxylin and eosin staining are standard. The handling of cytologic specimens, especially fine-needle aspirates, is much less standardized. A number of methods are available to prepare and fix fine-needle aspirates after the collection procedure and in the laboratory. Several stains (Diff-Quik, Papanicolaou, hematoxylin and eosin, and ultra-fast Papanicolaou stains), slide preparations (smear, cytospin, ThinPrep, and SurePath), cell block processing methods (at least 10 homebrews and automated processing), and fixatives (saline, alcohol-based, formalin, Roswell Park Memorial Institute [RPMI] culture medium, and CytoRich) are used. Furthermore, specimens are not triaged uniformly according to a protocol. Together, these factors produce inconsistent results across laboratories.
For optimal results, a standardized protocol and algorithm should be implemented in the laboratory. Along with improvements in tissue preservation, triaging the sample at the time of the procedure is increasingly important. In an ideal scenario, an interventionalist would collect a specimen according to a well-defined protocol. A cytologist—preferably someone onsite—would immediately assess the specimen’s adequacy, confirming the presence of diagnostic material and determining if there is enough material for any necessary ancillary studies. The interventionalist and the cytologist should communicate with each other at the time of the procedure, and the objectives of the sampling procedure should be specified to the extent possible. For example, if the initial small sample is needed only to confirm a diagnosis of malignant disease before definitive removal of the tumor, it is unnecessary to obtain additional samples because samples needed for advanced diagnostic studies could be obtained later during the surgical resection.
Techniques for the optimal triage and preparation of small specimens for diagnosis and ancillary studies are outlined in this chapter.
Core-Needle Biopsy Versus Fine-Needle Aspiration
The decision to perform a core-needle biopsy or fine-needle aspiration depends on various factors, including operator and pathologist preference, availability of rapid onsite evaluation (ROSE), risk of complications such as pneumothorax or hemorrhage, the possibility of tumor cell seeding, the type of lesion (epithelial or spindle cell), and the lesion’s location and size. To date, there has been no consensus on the preferred method. Critics of fine-needle aspiration question whether it is possible to obtain sufficient material for molecular diagnosis with this technique. In contrast, proponents of cytologic specimens cite evidence that sampling different areas of a neoplasm with fine-needle aspiration is superior to using core-needle biopsy to obtain a specimen from a single area. In a study published in 2012, it was reported that fine-needle aspiration and core-needle biopsy performed with ROSE provide equivalent results in terms of diagnosing a specific malignancy and obtaining sufficient material for ancillary studies (e.g., IHC and molecular testing) to guide tumor-specific treatment. Lastly, molecular testing results showed 100% concordance between cytologic samples obtained by fine-needle aspiration and histologic resections obtained by core-needle biopsy.
Some pathologists advocate a sequential approach to obtaining small specimens, namely, initial use of fine-needle aspiration followed by core-needle biopsy, if additional tissue is necessary. Improved results have been demonstrated in studies in which fine-needle aspiration and core-needle biopsy are used together compared with either procedure alone. A combined approach may be feasible for transthoracic CT-guided biopsy with use of a coaxial needle, which permits acquisition of either type of specimen while minimizing the number of punctures.
The two methods of obtaining small specimens may provide complementary information. For this reason, reviewing cases with paired cytologic and histologic specimens can be useful in rendering a specific and concordant diagnosis and minimizing the number of diagnoses of NSCLC not otherwise specified that can result from having samples with poor differentiation or scant cellularity.
Navigational Bronchoscopy for Sample Collection
With navigational bronchoscopy, a sample can be collected with either aspiration or core-needle biopsy forceps. Little has been published comparing the cellular yield of each method. In one study, it was reported that catheter aspiration was associated with a higher diagnostic yield than biopsy. The authors hypothesized that the back-and-forth motion of the catheter and the aspiration enabled access to target cells that may not be accessible by forceps biopsy. When both sampling procedures are going to be performed, experience has shown that it is better to conduct the aspiration first because doing so produces a less bloody sample without diluting the cells of interest.
Rapid ONSITE Evaluation
Advantages
One of the most effective ways to ensure appropriate management of fine-needle aspiration is with ROSE, which involves working with an experienced cytopathologist or cytotechnologist in an adjacent room at the time of the procedure. Several studies have demonstrated the usefulness of ROSE in optimizing the yield and efficiency of fine-needle aspiration and increasing its diagnostic accuracy.
Onsite assessment has many advantages. First, it is an integrated approach to diagnosis. The cytologist obtains the pertinent history and can correlate the morphologic features with clinical findings and imaging study results. A preliminary evaluation and diagnosis is valuable in patient management. For instance, when treatment is urgent or patients have travelled far for care, processing can be initiated when the sample arrives in the laboratory. Appointments with clinicians involved in treatment can be scheduled, and additional imaging studies or laboratory tests can be performed without delay.
By providing real-time feedback, ROSE decreases the number of false-negative diagnoses, which usually result from sampling error by the interventionalist. Well-defined sampling protocols for obtaining specimens may minimize false-negative results; an example is requiring documentation of the needle tip in the lesion. Sparse cellularity obscured by blood, inflammation, or foreign material in a cytologic specimen can contribute to false-negative results. When an onsite cytologist determines that the specimen is inadequate, a second sample can be obtained during the same session rather than having the patient return for another procedure.
Although imaging provides guidance and confirmation of the needle’s placement in the target, the needle may inadvertently catch nonneoplastic cells or elements as it traverses through other tissues. Many times a specimen obtained by EBUS biopsy appears grossly diagnostic but consists of bronchial cells, macrophages, mucus, cartilage, or blood clot. With CT-guided sampling of pleural-based lesions, mesothelial cells may be the main cellular component. A core may consist mostly of necrotic tissue or clotted blood. In the absence of ROSE, these samples may be misinterpreted as diagnostic specimens.
ROSE confirms the presence of adequate viable, nonnecrotic tissue. In a setting without ROSE, additional dedicated passes are needed to increase the likelihood of a definitive diagnosis. Relative to a protocol that requires a fixed number of passes, immediate assessment with ROSE may mean fewer passes are necessary. Avoiding unnecessary passes decreases the duration of anesthesia or sedation and reduces potential morbidity. Benefits of shorter biopsy time include rapid turnover of the procedure room and imaging facilities and fewer repeat procedures, resulting in cost savings.
With ROSE, the cytologist also is involved in the adequate preparation and triage of specimens. Based on the preliminary assessment, the specimen can be allocated for IHC or molecular testing for carcinomas, microbiologic cultures in cases of inflammation or granulomas, or flow cytometry for lymphoma assessment ( Fig. 19.2 ).

A biopsy or aspiration may be performed to determine whether a lymph node is negative for carcinoma, infection, or lymphoma; this scenario is common during EBUS staging procedures. With ROSE, a cytologist is able to assess whether the specimen is representative of a lymph node and whether a sufficient number of lymphocytes have been included to prevent a false-negative diagnosis and increase the negative predictive value.
A well-prepared specimen expedites final examination by limiting the number of intradepartmental second opinions, consults, and deliberation necessitated by sparse cellularity, poor preparation, or artifacts; instead, cytologists can focus their attention on diagnostic dilemmas. When ROSE and the final interpretation are performed by the same cytologist, less time is required overall because a portion of the specimen has already been previewed.
Disadvantages
Experience and training are necessary to prepare smears, assess a sample, and triage the specimen, and not all institutions have the resources or personnel to provide ROSE. ROSE can be time consuming: in addition to the procedure time, travel time to and from the procedure site can be substantial for large institutions or offsite practices. Given the time required for ROSE, reimbursement is low, and a pathologist can generate greater revenue and relative value units by examining slides under a microscope in his or her office.
Algorithm for Processing Small Samples
No standardized algorithm exists for processing small specimens, and few methods have been outlined. The goal of processing small specimens is to preserve as much material as possible for molecular testing, especially for patients with advanced lung adeno cancer. Ideally, each institution should engage a multidisciplinary team and determine best practices for managing small specimens. An algorithm and ROSE were used together to obtain sufficient cytologic material by EBUS-guided transbronchial needle aspiration for reflex molecular testing in 93% of patients with adenocarcinoma ( Fig. 19.3 and Box 19.1 ).
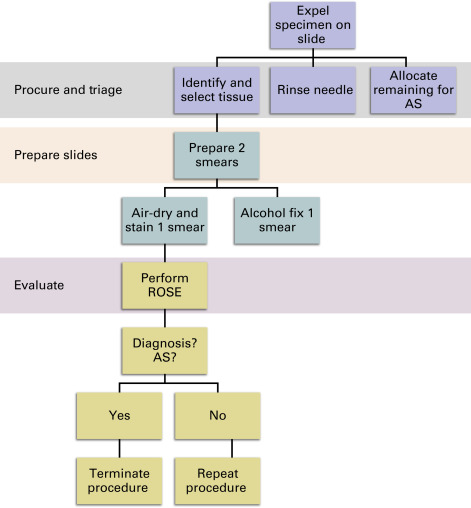
- 1.
For each pass of the needle, expel the specimen onto a single slide with a syringe. ( Fig. 19.3 ).
- a.
If clotting prevents the material from being expelled, use a stylet to dislodge the specimen.
- a.
- 2.
Identify diagnostic tissue particles, often tan or white specks but may vary depending on the nature of the lesion (e.g., mucoid material in cases of a mucinous carcinoma), and select them with the corner of a second slide ( Fig. 19.5 ).
- a.
When there is significant clot formation, gently press the specimen in between two slides to identify tissue particles.
- b.
A scant sample may allow for preparation of only one or two smears.
- a.
- 3.
For each pass, prepare two smears from the selected tissue particles ( Fig. 19.5 ).
- a.
Air-dry one smear and stain with Diff-Quik, or similar method, for ROSE.
- b.
Fix one smear in alcohol for Papanicolaou staining in the laboratory.
- a.
- 4.
Flush the needle and/or syringe to remove any remaining cells.
- a.
For CT-guided aspirations, rinse the needle and syringe in CytoLyt (or another preservative used by the laboratory).
- b.
For EBUS and/or EUS fine-needle aspiration, pass approximately 0.5 cc to 1 cc of saline through the needle into CytoLyt (or other preservative used by the laboratory).
- a.
- 5.
Place the remaining specimen in media appropriate for ancillary studies and/or cell block preparation.
- a.
Cell blocks can be made by allowing the specimen to clot on the expelled slide for a few minutes and then placing it into formalin. (Partial) clotting simplifies cell block processing.
- b.
Separate diagnostic material or more cellular elements from nondiagnostic ones (e.g., passes containing mostly blood) to prevent specimen dilution and improve the cellular yield of cell blocks.
- a.
- 6.
Perform ROSE.
- a.
Is there sufficient material for diagnosis?
- i.
If not, repeat steps 1–5.
- ii.
If yes, and ancillary studies are needed, determine whether the specimen includes sufficient material.
- iii.
If no, perform dedicated passes for ancillary studies.
- iv.
If yes, end the procedure.
- i.
- a.
CT, computed tomography; EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; ROSE, rapid onsite evaluation.
Managing Small Samples without Rapid ONSITE Evaluation
When ROSE is not possible, there are some strategies to facilitate good management of samples obtained with fine-needle aspiration, although no standardized protocols exist. Interventionalists can prepare slides for the pathologist’s review, either at the time of the procedure or afterward. Telepathology, in which a pathologist views prepared slides from a remote location, may be an alternative to ROSE. As technology improves, the role of telepathology is likely to increase, although additional studies are needed to determine best practices. Neither of these approaches ensures that an adequate specimen has been procured for ancillary testing or that the specimen will be triaged appropriately, however.
Performing a fixed number of aspirates per site (e.g., three) and a dedicated pass for cell block preparation or ancillary studies can enhance the cellular yield. However, additional samples cannot always be obtained, especially when doing so poses an increased risk to the patient. To minimize suboptimal smearing and specimen use, the specimen can be placed directly into a fixative for liquid-based cytologic examination, or a cell block can be prepared; the usefulness of this technique has not been formally studied, though. Above all, it is essential to define a process for handling specimens in coordination with the cytopathology laboratory.
Optimization and Triage
Optimal use of a specimen requires appropriate handling and triage, with attention to both the quantity and quality of the specimen. Even in the presence of abundant tissue, poor preparation and allocation can preclude definitive diagnosis and ancillary studies.
Optimization
Slide Preparation for Fine-Needle Aspirate
It is necessary to prepare only enough smears to make a diagnosis; the preparation of excessive smears can leave insufficient tissue for ancillary studies. Ideally for ROSE, only two smears should be prepared per pass: one air-dried for Diff-Quik staining and the second fixed in alcohol for Papanicolaou staining. Any remaining material can be allocated for cell block preparation or other ancillary studies ( Fig. 19.4 ). It is preferable to avoid thick smears. Not only do thin, even smears minimize tissue expenditure, they also allow for better visualization of diagnostic cells. Excess material and clots can be placed in a fixative or medium, which may obviate the need for additional passes for ancillary studies.