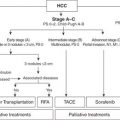
1.An R0 resection of both the primary lesion and any extrahepatic disease sites must be technically feasible.
2.At least two adjacent liver segments need to be spared.
3.Vascular inflow and outflow and biliary outflow to the remaining liver segments must be spared.
4.The FLR must be of adequate volume to account for any underlying hepatic dysfunction.
With expanded criteria of resectability for CRLM, a number of strategies for improving resectability have emerged, with the goal of increasing the number of patients eligible for hepatic resection for CRLM. These strategies fall into three broad categories: increasing hepatic reserve, decreasing tumor size, and using combined modality local therapy.
Portal vein embolization (PVE) has been used to increase hepatic reserve in patients undergoing hepatectomy for CRLM (Fig. 15.1). In patients with normal hepatic function, an FLR of 20% to 30% is required to ensure adequate hepatic regeneration and function following resection. Patients with underlying hepatic dysfunction require a larger FLR: Patients with steatosis or steatohepatitis require an FLR of 30% to 40%, and patients with cirrhosis require a 40% to 50% FLR. FLR can accurately be measured on CT or MRI using volumetric assessment. This is the most commonly used method for measuring FLR in Western centers and is used to identify patients who may benefit from preoperative PVE. Indocyanine green retention at 15 minutes has also been demonstrated to predict postoperative liver failure and mortality; however, the availability of this technique in Western centers is limited. Preoperative PVE is a technique that involves embolization of the portal vein with cyanoacrylate, gelatin microspheres, polyvinyl alcohol, or coils. Embolization of the tumor-bearing liver induces hypertrophy of the contralateral liver and thus increases the FLR volume. PVE is well tolerated with a low complication rate (<5%) and normally results in an absolute increase in FLR of approximately 8% to 16% depending on the degree of underlying hepatic dysfunction. The timing and degree of hypertrophy in response to PVE may help guide patient selection for operation.
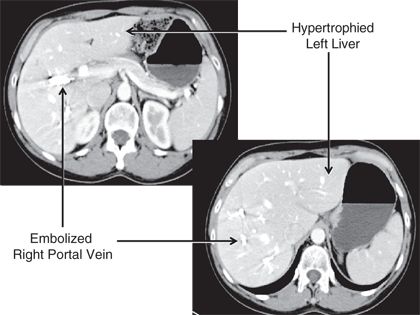
FIGURE 16.1 CT scan of the liver following PVE of the right portal vein; note the hypertrophy of the left liver.
A two-stage hepatectomy is a surgical approach that may be used in patients with extensive disease involving both sides of the liver (Fig. 15.2). In a subset of patients, clearance of all disease is often not possible in one operation due to concerns of an inadequate FLR. When the CRLM are bilateral, PVE is often not ideal as embolization of the entire tumor-bearing liver is not feasible. In these circumstances, a two-stage approach may be possible. With the two-stage approach, the disease in one hemiliver is cleared and the contralateral portal vein is occluded either by portal vein ligation during this first operation or by PVE performed postoperatively. The remnant portion of the liver that has been “cleared” is then allowed to hypertrophy, and then the patient is brought back to the operating room 4 to 6 weeks later to resect the remaining disease in the liver. When performing a two-stage hepatectomy, most surgeons advocate initial resection of minor disease (three or fewer segments) followed by resection of major disease (greater than three segments) at the second operation. Doing the minor liver resection first will spare a subset of patients the morbidity of a major hepatic resection, as 20% to 30% of patients will never undergo the second stage due to disease progression or worsening performance status. Of note, patients undergoing a two-stage approach can safely be treated with chemotherapy between stages without impairing hypertrophy of the FLR.
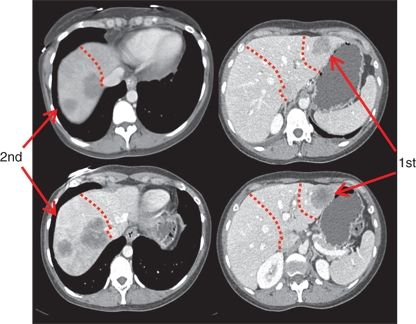
FIGURE 16.2 Depiction of a two-stage hepatectomy. The first stage was a segmentectomy of the left lateral bisector; following PVE, the patient was brought back to the operating room 6 weeks later for the second stage, which was a right hepatectomy.
Improvements in chemotherapy for patients with CRC have substantially improved tumor response rates in patients with CRLM (Fig. 15.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree