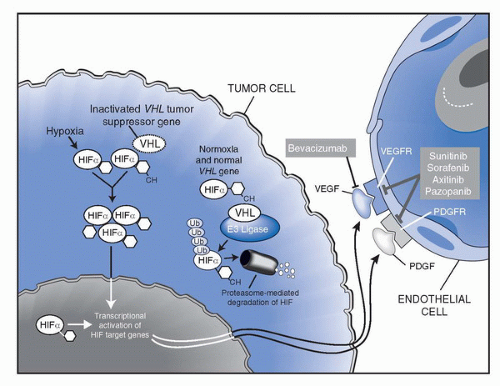
VEGF (28). The clinical utility of bevacizumab in mRCC was initially investigated in a randomized phase II trial in which 116 patients with mRCC with clear cell histology were randomized to receive placebo, low-dose (3 mg/kg) bevacizumab or high-dose (10 mg/kg) bevacizumab given intravenously every 2 weeks (29). All patients had prior disease progression despite at least one systemic treatment regimen; the vast majority (93%) had received prior IL-2. Groups were balanced using established prognostic factors (3). The study was designed to detect a twofold time to disease progression (TTP) increase with either dose of bevacizumab versus placebo, with results showing a 4.8 versus 2.5 months PFS favoring the high-dose bevacizumab arm versus placebo; (p < 0.001 by log-rank test). There were four partial responses, all in the high-dose bevacizumab arm (4/39; 10% ORR). Common toxicity included grade (G) 1/2 hypertension (HTN) and proteinuria, more commonly seen in the high-dose bevacizumab arm. All toxicities were reversible with cessation of therapy.
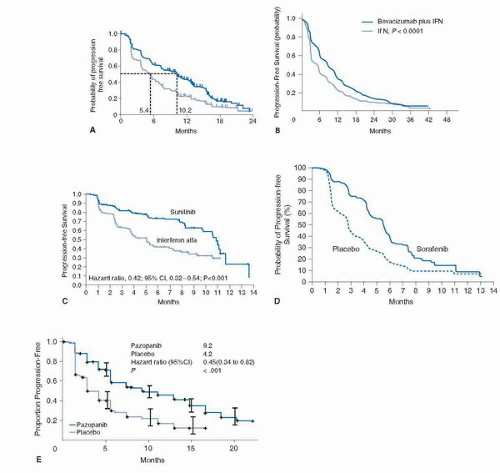
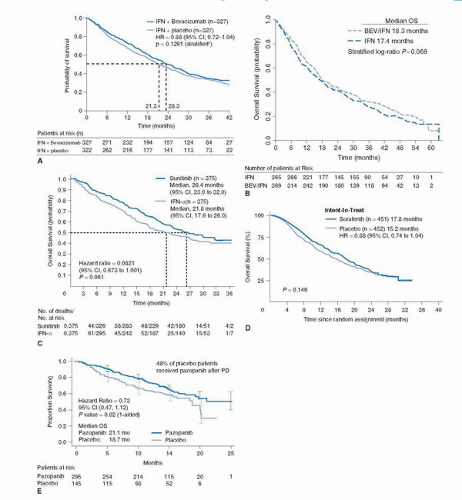
to detect an OS improvement from 13 to 17 months with PFS, ORR, and safety as secondary endpoints. Due to the change in standard of care and the availability of other active VEGF inhibitors that precluded reaching the anticipated OS endpoint, the study was amended and unblinded at the time of final PFS analysis. The median PFS observed was 10.2 months in the bevacizumab plus IFN-α group, compared with 5.4 months in the control group (HR 0.63, 95% CI 0.52-0.75; p = 0.0001) (Fig. 45A.2A). The improvement of PFS was largely confined to good- and intermediate-risk patients. (Good risk: 12.9 vs. 7.6 months; intermediate risk: 10.2 vs. 4.5 months; poor risk: 2.2 vs. 2.1 months.) A significant ORR difference was also observed in favor of the bevacizumab-treated patients (31% vs. 13%; p < 0.0001). The final median OS was 23.3 months in the bevacizumab arm compared to 21.3 for the IFN-α plus placebo-treated arm (HR 0.86, 95% CI 0.72-1.04; stratified log-rank test p = 0.1291) (43) (Fig. 45A.3A).
TABLE 45A.1 RANDOMIZED PHASE III DATA OF VEGF INHIBITORS IN MRCC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
with bevacizumab remains unknown. Notwithstanding the fact that a significant percentage of patients receiving the bevacizumab-containing regimen in both phase 3 trials required dose modifications of IFN-α (41% AVOREN and 37% CALGB90206) a recent exploratory analysis of the European study would suggest that the improvement of PFS observed with the addition of the VEGF antibody to IFN-α appears to be maintained in spite of the need for IFN-α dose reductions (10.2 months with full dose vs. 12.4 months in patients who required a reduced dose of IFN-α) (41). Given the lack of dose response for interferon, it is possible that lower interferon doses in this combination can reduce toxic effects and preserve efficacy. Such a hypothesis requires prospective testing.
combination of VEGF-R inhibitors with bevacizumab (44,45,46,47). A phase I study evaluated the maximum-tolerated dose (MTD) and safety of the combination of bevacizumab and sorafenib in 39 patients with advanced refractory malignancies (44). Among these, only three patients were mRCC. Treatment included sorafenib 200 mg orally twice daily and bevacizumab intravenously at 5 mg/kg (dose level [DL] 1 or 10 mg/kg [DL2]) every 2 weeks with a preplanned level 3 where sorafenib was escalated to 400 mg orally twice daily. These doses were below approved single-agent dose and selected because of concerns of potentiating toxicity. The most common adverse events (AEs) included HTN, hand-foot syndrome (HFS), diarrhea, transaminitis, and fatigue. A dose-limiting toxicity (DLT) of recurrent G2 HFS was noted in the first DL. The unexpected severity of toxicity seen prevented from full dose escalation suggesting that this combination may not be tolerable long term and therefore alternate sorafenib dosing schedules are currently undergoing evaluation (45). Recently, the results of two separate phase I trials evaluating the combination of bevacizumab plus sunitinib were reported (46,47). While one trial (46) evaluated the MTD and safety of the combination exclusively in mRCC (n = 26), the second trial (47) evaluated the same regimen in 38 patients with refractory solid malignancies. Among these, 16% (6/38) were mRCC. In both trials, treatment was administered in 42-day cycles, during which patients received oral sunitinib once daily from days 1 to 28 followed by 14 days off and bevacizumab intravenously every 2 weeks starting of day 0 or 1 of treatment. Despite differences in dose escalation design among the studies, the MTD of the combination was defined as 50 mg sunitinib and 10 mg/kg bevacizumab. Although antitumor effect was observed in mRCC and notably in other advanced solid tumors typically refractory to traditional chemotherapy, the common denominator for both trials was toxicity. In the exclusive RCC trial, the most frequently reported AEs (any grade [G]) for all patients included fatigue (92%), HTN (92%), proteinuria (88%), diarrhea (76%), hand-foot-skin reaction (72%), and bleeding (72%). The most frequently reported G3 to 4 AEs included HTN (60%), proteinuria (36%), and thrombocytopenia (24%). Similarly, in the broad phase I study, G3 or greater toxicity was observed in 82% of patients including most commonly fatigue (64%), HTN (51%), proteinuria (33%), thrombocytopenia (31%), and anorexia (26%). In both trials, the majority of G3 to 4 events occurred at the highest dose. A major toxicity concern was the finding of a microangiopathic hemolytic anemia (MAHA)-like clinical picture observed in five patients who have reached full dose of both sunitinib and bevacizumab. Two of these patients also developed reversible posterior leukoencephalopathy syndrome (RPLS). Although none of these patients required treatment with plamapheresis and their clinical symptoms and laboratory features reversed upon treatment discontinuation, this appears to be a severe toxicity when these two agents are combined in RCC.
 Sunitinib-treated patients had a greater median OS when compared with the IFN-α group (26.4 months; 95% CI, 23.0-32.9 months; vs. 21.8 months; 95% CI, 17.9-26.9 months, respectively; HR 0.821; 95% CI, 0.673-1.001; p = 0.051) (Fig. 45A.3C) based on the primary analysis of the unstratified log-rank test (p = 0.013 using the unstratified Wilcoxon test). By stratified log-rank test, the HR was 0.818 (95% CI, 0.669-0.999; p = 0.049). The benefit of sunitinib over IFN-α was observed in all mRCC patients regardless of their MSKCC risk classification. More than 50% of patients in both arms of this trial went to receive subsequent treatment with a VEGF-targeted agent including sunitinib, thus the lack of statistical significance observed in the prespecified OS analysis. The results of this trial have positioned sunitinib as a standard front-line therapy for mRCC patients.
Sunitinib-treated patients had a greater median OS when compared with the IFN-α group (26.4 months; 95% CI, 23.0-32.9 months; vs. 21.8 months; 95% CI, 17.9-26.9 months, respectively; HR 0.821; 95% CI, 0.673-1.001; p = 0.051) (Fig. 45A.3C) based on the primary analysis of the unstratified log-rank test (p = 0.013 using the unstratified Wilcoxon test). By stratified log-rank test, the HR was 0.818 (95% CI, 0.669-0.999; p = 0.049). The benefit of sunitinib over IFN-α was observed in all mRCC patients regardless of their MSKCC risk classification. More than 50% of patients in both arms of this trial went to receive subsequent treatment with a VEGF-targeted agent including sunitinib, thus the lack of statistical significance observed in the prespecified OS analysis. The results of this trial have positioned sunitinib as a standard front-line therapy for mRCC patients.Similarly, over 30% of patients (n = 1,418) in the study were older than 65. Among all evaluable patients (3,464/4,564) the ORR observed was 17% (n = 603). Similarly, the ORR for patients with non-clear cell histology (n = 437) was 11%. The median PFS for the entire cohort was 10.9 months (95% CI 10.3-11.2) and the median OS was 18·4 months (17.4-19.2).
the median OS in the entire cohort was 50 weeks (95% CI, 46-52; censorship rate, 63%). The efficacy and safety results were similar across the subgroups.
has been shown to inhibit angiogenesis, vascular permeability, and blood flow (74). Although axitinib inhibits PDGF receptors and KIT with nanomolar in vitro potencies, based on pharmacokinetic/pharmacodynamic analysis, axitinib acts primarily as a VEGFR tyrosine kinase inhibitor at the current clinical exposure. A phase II single-arm, multicenter trial of axitinib in cytokine refractory mRCC patients (n = 52) demonstrated an ORR of 44% (95% CI 30.5-58.7) (75). The median TTP was 15.7 months (8.4-23.4, range 0.03-31.5). Similar to other small molecule tyrosine kinase inhibitors, most toxicity was grade 1 or 2 and included gastrointestinal, dermatologic, fatigue, HTN, and proteinuria. To determine the activity of this agent in patients with VEGF-refractory mRCC, an additional study enrolled 62 patients who developed PD while on sorafenib treatment (76). Since the previous study indicates variable drug levels in patients (75), the axitinib dose was increased in a step-wise fashion from 5 mg twice daily to 7 mg twice daily and then to 10 mg twice daily, in the absence of predefined toxicities. Sixteen patients (25.8%) had received one additional prior therapy regimen, and 46 patients (74.2%) had been treated with at least two prior regimens. Approximately 20% of patients have received prior therapy with sunitinib. The ORR observed in the study was 22.6% and the median duration of response was 17.5 months (95% CI, 7.4 months to not estimable). Objective responses were observed regardless of the axitinib dose received. With a median follow-up of 22.7 months, median PFS for the entire study population was 7.4 months (95% CI, 6.7-11.0 months). The safety profile of axitinib in this trial was consistent with previously reported findings (74,75), although a higher incidence of HFS was noted. Currently, a randomized phase III trial evaluating this agent against sorafenib in patients with treatment-refractory disease has completed accrual with results pending.
TABLE 45A.2 OVERVIEW OF COMMON TOXICITIES OBSERVED WITH VEGF INHIBITORS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
however, is often a cause for dose modification and even drug discontinuation. The underlying mechanism for developing HFS associated with sorafenib and sunitinib has not been determined; however, the focal occurrence of the skin lesions observed with sunitinib or sorafenib compared with the traditional HFS or palmar plantar erythro-dysesthesia (PPE) associated with classic chemotherapy suggests that blood vessels under pressure points may be more prone to damage due to the VEGF/PDGF-R inhibitory activity of these agents (77).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree