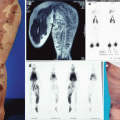
Fig. 51.1
Classification of lymphedema (C. Campisi, 2001)
In order to provide a comprehensive classification system of lymphedema that encompasses immunohistopathological criteria, level of clinically evident edema, lymphoscintigraphic findings, and level of physical disability, we developed a three-stage model (Fig. 51.2) [9, 12, 13]. In clinical practice, stages IA, IB, IIA, and early IIB can be considered as early manifestations of disease and late IIB, IIIA, and IIIB stages to be chronic and advanced. It should be noted that lymphedema is a progressive disease and can move rapidly between the stages without adequate treatment [14–17].
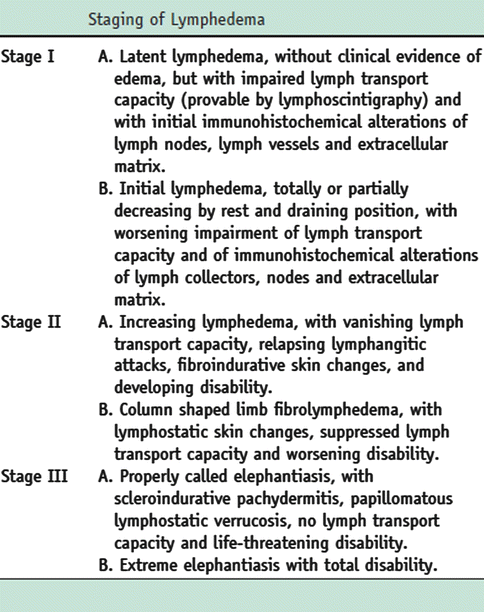

Fig. 51.2
Staging of lymphedema, based on immunohistological criteria, lymphoscintigraphic findings, clinical symptoms, and grade of physical disability (C. Campisi, August 2009)
General Considerations of the Surgical Treatment of Lymphedema
Refractory lymphedema unresponsive to conservative treatment measures may be appropriately managed by surgical means. Indications for surgery include insufficient volume reduction by appropriate conservative methods (less than 50 % reduction), recurrent lymphangitis or erysipelas episodes, intractable pain or discomfort usually associated with excess swelling and inflammation, loss of limb function and increasing disability, and patient dissatisfaction with previous treatment outcomes and willingness to proceed with surgery.
The initial derivative microsurgical approaches involved lymph node-venous shunts, but, aside from India where these have been used frequently in the treatment of lymphatic filariasis, these approaches have been largely superceded by more refined and effective techniques. Lymph node-venous shunts had a high rate of anastomotic closure due to the thrombogenic effect of the lymph nodal pulp on the blood and the frequent re-endothelialization of the lymph node surface [18]. Worldwide, surgeons moved to anastomosing lymphatic vessels directly into the veins in order to increase the long-term efficacy of the procedures [19].
The lymphatic-venous anastomosis technique involves joining an appropriate lymphatic vessel to a collateral branch of a main vein, ensuring that the vein has competent valvular function and continence. This is essential to prevent reflux of the blood into the lymphatic vessel and thereby thrombosis of the anastomosis [12].
Clinical Experience and Surgical Techniques
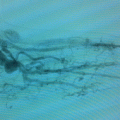
The microsurgical interventions involved multiple lymphatic-venous anastomoses (LVA). Blue patent violet dye (BPV; a sodium or calcium salt of diethylammonium hydroxide) is used intraoperatively to identify properly functioning lymphatic vessels at the surgical incision. Healthy-appearing lymphatic vessels at this site are selected and introduced directly into the vein segment with a U-shaped stitch (using 8/0–10/0 Prolene sutures depending on the caliber of the vessels). These are then secured by additional stitches between the perilymphatic adipose tissue and the vein border. The passage of the blue-stained lymphatic fluid into the vein, evident under the operating microscope, verifies the patency of the anastomosis (Fig. 51.3).

Fig. 51.3
LVA: the passage of blue lymph into the vein branch can be seen under the operating microscope, verifying the patency of the anastomosis
For patients with lower-limb lymphedema, multiple LVAs are applied in the subinguinal region. Superficial lymphatic-lymph nodal structures are identified and isolated, and all available and healthy afferent lymphatic collectors are used for the anastomoses. Samples of lymph nodes, vessels, vein segments, and adjacent tissues are subjected to histopathological analyses. Common findings in primary lower-limb lymphedema are significant thickening of the nodal capsule and varying levels of nodal fibrosclerosis. Afferent lymphatic vessels are usually normal, indicating a proximal obstruction in lymphatic flow.
For patients with upper-limb lymphedema, multiple LVAs are performed at the middle third of the volar surface of the arm. Both superficial and deep lymphatic collectors identified by BPV are used. Deep lymphatics are typically found in the vicinity of the humeral artery, vein, and median nerve. A patent branch of one of the humeral veins is used in the application of microsurgical anastomoses.
Lymphoscintigraphy is performed in all cases as part of the diagnostic workup to select appropriate candidates for surgery based on evidence of proximal obstruction of lymphatic flow. Lymphoscintigraphy is performed with either 99mTc-labeled antimony sulfur colloid or 99mTc-nanocolloid human serum albumin (90 % of the particles of >80 nm in size) injected into the interdigital space of the toes or fingers of both limbs in the affected region. It is essential to ensure that the edema is of lymphatic origin. Lymphoscintigraphy also provides important etiological and pathophysiological information about the nature of the disease. In the last 2 years of our experience, evaluation of the superficial lymphatic pathways distal to the surgical site in the affected limb has also been performed using the photodynamic eye (PDE) method with injection of indocyanine green fluorescence. The simultaneous use of PDE with BPV gives two methods of visualizing the lymphatic flow and confirmation of patency of the anastomoses intraoperatively [20, 21]. PDE can also be used postoperatively, in addition to lymphoscintigraphy, to verify the long-term stability of the surgical outcomes.
Primary lymphedemas typically involve lymph node dysplasias (LAD II according to Papendieck’s classifications [22]) with hypoplastic lymph nodes associated with sinus histiocytosis and a thick fibrous capsule and microlymphangioadenomyomatosis. In these cases, obstruction to lymphatic flow is evident in the changes in the afferent lymphatic collectors: these are dilated, swollen, and tortuous with thickened walls and reduced numbers of smooth muscle cells, which are fragmented by numerous fibrotic elements. In our clinical experience, the majority of the peripheral lymphedemas treated by microsurgical means were at stages IIA (39 %) and IIB (52 %), where a minority were at other stages (3 % at stage IB and 6 % at stages IIIA and B; according to the Campisi staging system for lymphedema [9, 12, 13]).
Echo Doppler is performed in all cases to exclude venous-only causes of edema and also to identify any venous anomalies associated with the lymphedema, such as phlebolymphedema. In most cases, the venous anomalies can be corrected contemporaneously, such as valvuloplasty for venous insufficiency. In other cases, venous disease is an indication for performing a venous graft between the lymphatic collectors above and below the site of lymphatic obstruction, as the valvular incompetency of the diseased veins would compromise the efficacy of the anastomoses if LVA was performed instead. This venous bridge type of graft is called a lymphatic-venous-lymphatic anastomosis (LVLA). Competent venous segments are harvested from the same operative site or can be taken from the forearm (usually the cephalic vein). The length of the grafted vein section varies from 7 to 15 cm as required. It is essential to collect several lymphatic collectors to join to the distal end of the vein to ensure that the vein segment is filled with sufficient lymph and to avoid closure due to subsequent fibrosis development. The competent valves of the vein segment are vital for directing the flow of lymph in the correct direction and to avoid gravitational backflow or reflux. As with the LVA, the lymphatic collectors are directly introduced into the vein cut ends by means of a U-shaped stitch, which is then stabilized with additional peripheral stitches (Fig. 51.4).
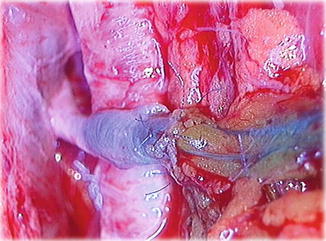

Fig. 51.4
A schematic drawing of the derivative multiple LVA and the reconstructive multiple LVLA technique with the interposition of an autologous vein graft between lymphatics above and below the obstacle to the lymph flow (C. Campisi, 1982)
Results
Clinical outcomes improved earlier that microsurgical techniques are applied in the treatment of peripheral lymphedema, due to the absence of, or minimal, fibrosclerotic tissue changes in the lymph vessel walls and surrounding tissues. Compared to preoperative conditions, patients obtained significant reductions in excess limb volume of over 84 %, with an average of 69 % as measured by limb water volumetry and circumference. These results were stable over an average of 10 years of follow-up. Over 86 % of patients with stages I and IIA gradually stopped using conservative therapies over the length of the follow-up period. In patients with more advanced lymphedema (stages IIB, IIIA, and IIIB), 42 % could decrease the frequency of physical therapies in the long term. In all patients, the frequency of DLA attacks considerably reduced by over 91 %, compared to preoperative conditions.
There were no immediate postoperative complications, such as postoperative infections, postoperative lymphorrhea, or worsening of edema. Most recently, patency verification was also performed postoperatively using the PDE method with indocyanine green fluorescence. This method allows visualization of the superficial lymphatic pathways and is valuable to confirm the significant reduction in dermal backflow of lymph after microsurgery. When PDE is used immediately after surgery, it is possible to verify the anastomosis patency and provide evidence that no thrombosis has occurred.
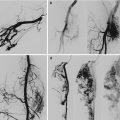
Lymphoscintigraphy was used to verify the patency of the microanastomoses in the long term by direct and indirect methods (Fig. 51.5). These included the following:
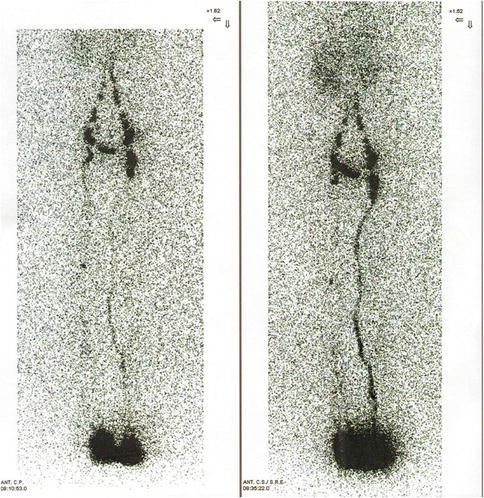

Fig. 51.5
Preoperative lymphoscintigraphy in a patient affected by primary lymphedema of the left leg (left). Postoperative lymphoscintigraphy shows the appearance of preferential lymphatic pathways into the inguinal region (right)
Reduced dermal backflow of the tracer and the appearance of preferential lymphatic pathways not evident preoperatively
The disappearance of the tracer at the site of the lymphatic-venous anastomoses, indicating the passage of lymph into the bloodstream
Earlier liver uptake of tracer, compared to preoperative parameters, taken as indirect evidence of the passage of lymph in the bloodstream
Additional Considerations
Combined physical therapy should be the initial treatment for peripheral lymphedema and is best conducted in specialized treatment centers. The timing of the surgical intervention is important. In the case of established lymphedema (stages IB and onwards), surgical treatment should be implemented as soon as there is no further reduction in limb volume obtained by conservative methods or earlier if there are recurrent lymphangitic attacks that act to worsen the lymph transport [23]. Microsurgery applied at this point provides further amelioration in the condition [24, 25]. Early application of microsurgery techniques is efficacious in preventing the development of lymphedema in certain cases and is discussed further in a later section.
The ideal indications for microsurgical intervention, in our experience, include relatively early stages of disease (stages IB, IIA, and early IIB); lymphoscintigraphy patterns showing low inguinal or axillary nodal uptake of the tracer and minimal or absent passage of the tracer beyond this proximal area; excellent patient compliance with all treatment aspects; and access to a well-organized lymphedema treatment center where the patient can be referred to a center of lymphatic surgery when a specialized surgery is required.
In more advanced cases (late IIB, IIIA, IIIB), where lymphoscintigraphy shows no visualization of lymphatic channels and regional lymph nodes, it is necessary to reduce the stage of lymphedema by intensive conservative methods prior to surgery, in order to reduce the clinical stage of the disease and best prepare the limb for surgery. Lymphatic microsurgery has an important role in the treatment of advanced lymphedema where addressing the chronic lymph stasis helps with edema reduction but also is likely to improve the immune function in the affected limb [15], as recent research indicates that chronic lymph stasis is associated with reduced immune responses to infection. These patients need to be followed closely in the postoperative phase and to adhere to the regimen of complete lymphedema functional therapy, ClyFT [26]; this is essential to maintain the results obtained by the microsurgery and to continue to improve the long-term clinical outcome (Fig. 51.6). As advanced cases of lymphedema are associated with fibrotic adipose tissue deposits due to chronic lymph stasis and inflammation, an additional surgical approach may be applied and is discussed in a further section.
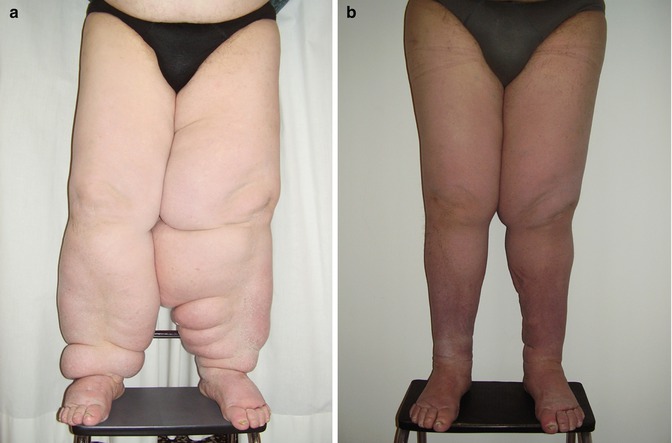

Fig. 51.6
Preoperative photo of a patient with advanced lower-limb lymphedema (a). Postoperative photo 1 year from surgery (b)
Regardless of the stage of disease, if patient compliance to the treatment program is poor, then the outcome may be less than satisfactory. Relative contradictions to lymphatic microsurgery are few and are represented by cases of lymphatic-lymph node aplasia (extremely rare), diffuse metastatic disease, and very advanced stage of lymphedema (stage IIIB) totally unresponsive to conservative therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree