Lung Cancer
INCIDENCE AND ETIOLOGY
Lung cancer is the most common cancer in the world, with about 1.2 million new cases per year ( ). The estimate for new cases of lung cancer in the United States in men for 2009 is 129,710 and among women, 107,280 ( ). Lung cancer will still be the leading cause of death from malignancy in both sexes in the United States, resulting in 71,550 estimated deaths in women in 2009 and 92,240 deaths in men. Lung cancer is responsible for 25% of all cancer deaths and for 5% of all deaths in the United States. The vast majority of cases (85% or more) are due to chronic cigarette smoking. Other etiologic factors include asbestos (shipyard workers, insulators, etc.), radon gas (underground mining, etc.), ionizing radiation, and certain industrial agents and compounds (chloromethyl ether, arsenic, nickel-cadmium, and chromium). Tobacco smoking is thought to be synergistic with the latter elements. Tobacco smoke contains oxidants that are believed to be important in biologic damage of DNA, proteins, and lipids, leading to lung cancer. Genetic abnormalities as well as underlying lung disease (chronic obstructive pulmonary disease) also predispose patients to lung cancer.
Whereas lung cancer is one of the easiest cancers to prevent, it is one of the most difficult to cure, as a result of the early dissemination in many cases and therapeutically refractory disease when metastases occur. The overall rate of cure for all patients is about 16%, although there is a wide range of cure rates related mainly to stage of disease. If a patient survives the initial cancer the risk of subsequent lung cancer increases to 3% to 7% per year. By discontinuing cigarette smoking, the patient lowers the risk for primary lung cancer as well as subsequent cancer, but 15–20 years must pass before the risk approaches that of nonsmokers. Still, at 15 years after quitting smoking the risk is 1.5 times that of a person who never smoked. Passive smoking accounts for 3% to 5% of all cases of lung cancer. According to the U.S. Surgeon General, 5000–10,000 of the 160,000 deaths due to lung cancer each year occur in patients exposed to sidestream smoke.
LUNG CANCER, GENDER, AND AGE
Lung cancer rates in women have risen dramatically, both worldwide and in the United States. Population-based data by the National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) Program have shown that the age-adjusted rate for lung cancer for all race/sex groups has risen sharply since 1950. The incidence started rising in the mid-1930s and overtook breast cancer as the leading cause of cancer deaths among women in the late 1980s. This observation correlates with the increase in the number of women who smoke. The male-to-female ratio is 3.47 in patients over 45 years of age and 1.7 in patients younger than 45. Adenocarcinoma is the most common type in young patients and squamous cell carcinoma in the older age group. Notably, adenocarcinoma is increasing in women, especially nonsmokers. Reasons for this are multiple, including genetic factors, increased susceptibility to lung cancer carcinogens as compared with men, and secondary or passive smoking exposure ( ).
HISTOPATHOLOGY
Over 95% of lung neoplasms are of epithelial origin (carcinomas), comprising four main types (see below) ( Table 5.4 ). Based on clinical features and biologic properties from studies of cultured malignant cells, these carcinomas can be separated into two major categories: non–small cell lung cancer (squamous cell, adenocarcinoma, and undifferentiated large cell types) and small cell lung cancer ( ). About 5% of lung cancers are composed of rare mixed epithelial types or neoplasms arising from bronchial glands and other tissues ( Table 5.4 ).
| Type | Subtype * |
|---|---|
| Non–small cell carcinoma (85%) |
|
| Small cell carcinoma (10% to 15%) |
|
| Others (5%) |
|
* WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated.
† World Health Organization subtypes: acinar, papillary, bronchioloalveolar, solid with mucin production, mixed and variants.
Preinvasive Lesions
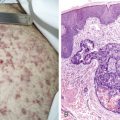
In the new World Health Organization (WHO)/International Association for the Study of Lung Cancer (IASLC) 1999 classification, preinvasive lesions include squamous dysplasia/carcinoma in situ (leading to squamous cell carcinoma), atypical adenomatous hyperplasia (characterized by discrete ill-defined bronchioloalveolar proliferation, leading to adenocarcinomas), and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (characterized by proliferation of neuroendocrine cells throughout the peripheral airways, leading to carcinoids) ( ).
Non–Small Cell Lung Cancer
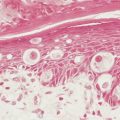
Non–small cell lung cancer (NSCLC) comprises about 85% of all lung cancers. They are subdivided into three groups. Squamous cell carcinoma (SCC) is characterized by keratin formation (cytokeratin proteins are intermediate filaments). Keratin may appear under the light microscope as “keratin pearls” (see Fig. 5.6 ) or as desmosomes (a type of tight junction seen as “intercellular bridges”; Fig. 5.6 ). The incidence of this type of lung cancer is decreasing in the United States. Most SCCs arise from the central or proximal tracheal-bronchial tree in areas of squamous cell metaplasia, dysplasia, and carcinoma in situ. SCCs grow slowly and tend to cavitate in about 20% of cases. About one third of SCCs are poorly differentiated and, as such, show a greater potential for distant spread, especially to the liver and small intestine. Poorly differentiated SCCs may acquire a spindle cell morphology that may mimic a sarcoma; identification of such tumors is based on finding a transition zone between the epithelial-appearing tumor cells and the spindle cells and/or on the demonstration of keratin in the spindle cells by immunohistochemistry.
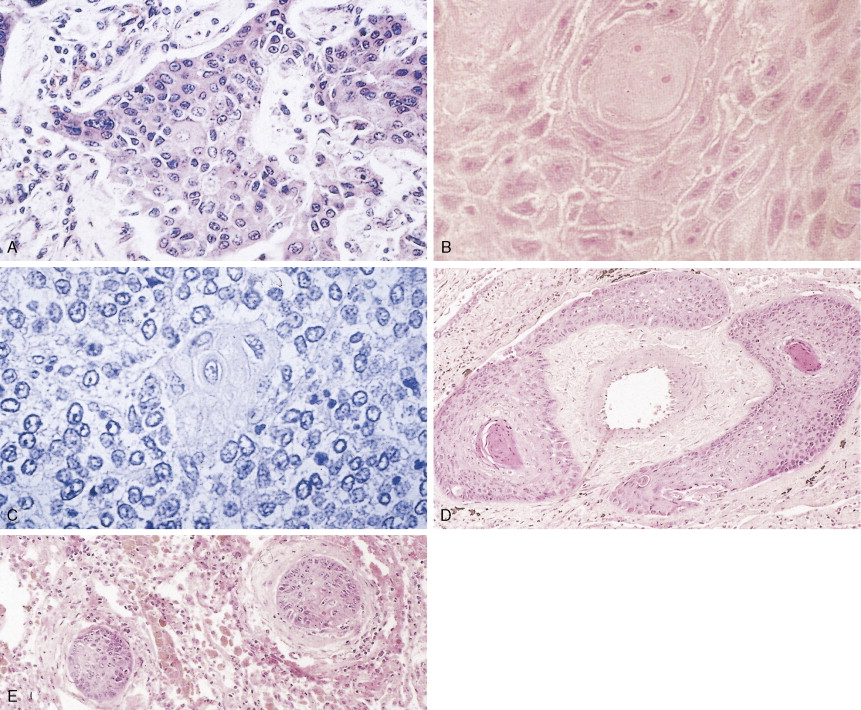
Adenocarcinoma is characterized by definite gland formation (well and moderately differentiated) or by the presence of mucus production in a solid tumor (poorly differentiated) as determined by mucin stains (mucicarmine and D-PAS) (see Fig. 5.10 ). Adenocarcinomas are increasing in frequency in the United States: at most medical centers they are now more common than squamous cell carcinomas. In about two thirds of cases adenocarcinomas originate in peripheral airways and alveoli. Classically, they were thought to arise from scars (“scar carcinoma”). This view is no longer accepted: the “scar tissue” (desmoplasia) is now thought to be induced by the neoplastic cells (see Fig. 5.9 ). Around one third of cases arise centrally, in larger bronchi, from either the surface epithelium or the submucosal glands. Adenocarcinomas frequently present as subpleural nodules, often with a malignant effusion. These cases must be differentiated by means of special stains from malignant mesothelioma, which lacks mucin (see Table 6.1 ). Metastases from adenocarcinoma to distant sites occur early (e.g., before symptoms or diagnosis) in most patients. Adenocarcinoma arising from sites other than lung can also look very similar to adenocarcinoma arising in the lung. Cytokeratins (7 vs 20) can aid in distinguishing adenocarcinoma from lung as opposed to other sites (in the case of lung, cytokeratin 7 is usually positive and cytokeratin 20 usually negative). TTF-1 (thyroid transcription factor-1) marker is found in adenocarcinoma of the lung and thyroid cancer and is useful in the differential diagnosis of metastatic adenocarcinoma from an unknown primary site (see Table 5.13 ) ( ).

| CK7 | CK20 | TTF-1 | CDX-2 | SMAD4 | P63 | |
|---|---|---|---|---|---|---|
| Lung adenocarcinoma | + | – | + (nuclear) | – | + | – |
| Lung squamous carcinoma | + | –/+ | –/+ | – | + | + |
| Ovarian cancer | + | – | – | – | + | – |
| Gastric cancer | +/– | +/– | – | +/– | + | – |
| Pancreatic cancer | + | + | – | +/– | – | –/+ |
| Appendiceal cancer | –/+ | + | – | + | + | – |
| Colonic cancer | – | + | – | + | + | – |
| Uterine cancer | + | – | – | – | + | – |
* Results may differ due to variable expression of the markers. Courtesy of Waichin Foo, M.D., Ph.D., Department of Pathology, Brigham and Women’s Hospital.
† CK7, cytokeratin 7; CK20, cytokeratin 20; TTF-1, thyroid transcription factor 1; CDX-2, nuclear transcription factor involved in intestinal development; SMAD, 4 transcriptional regulator expressed in normal tissues; P63, nuclear transcription factor.
‡ Interpretation: +, mostly positive stain; –, mostly negative stain; –/+, either positive or negative stain; SMADA: +, means retained; ndash;, means lost.
|
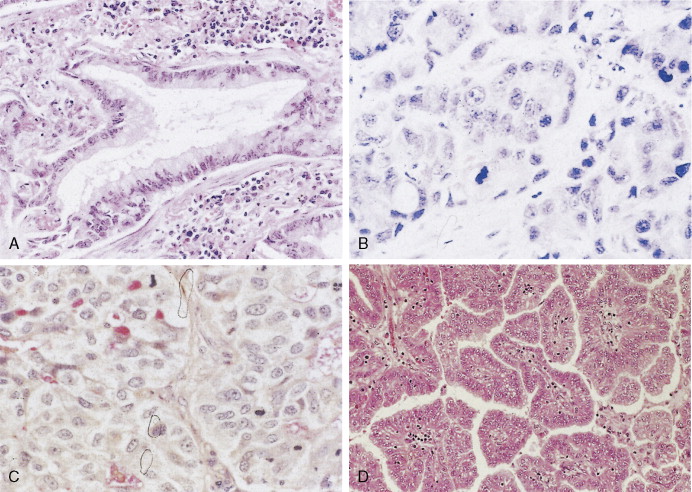
Bronchioloalveolar carcinoma (BAC) is a subtype of well-differentiated adenocarcinoma, constituting about 3% of cases (pure type) as compared with 20% for mixed types. It is increasing in frequency and is the one subtype of lung carcinoma (in addition to carcinoid tumors) that is not strongly associated with cigarette smoking. BAC arises from the peripheral bronchioles or alveoli. About 50% of BACs are mucin-secreting tumors consisting of tall columnar cells, whereas the remaining 40% have little or no mucin and consist of peg-shaped (“hobnail”) cells with variable degrees of pleomorphism. They are thought to arise from Clara cells or type II pneumocytes, respectively. Some adenocarcinomas may contain a small proportion of tumor cells with BAC morphology, typically in the periphery of the tumor. It is, however, generally designated as adenocarcinoma with BAC features. In the World Health Organization (WHO) classification, true BAC has growth in a lepidic fashion with lack of invasive growth ( ). BAC tends to spread throughout air passages while preserving (or recapitulating) the septal and lobular architecture. The tumor is slow-growing and usually metastasizes late in the course of the disease. It may induce a characteristic voluminous clear sputum production (bronchorrhea). Prognosis is related to stage of disease, but because BAC may be mistaken for chronic infection or diffuse interstitial disease, there may be a long delay in diagnosis.
Undifferentiated large cell carcinoma is characterized by large cells with vesicular nuclei, prominent eosinophilic nucleoli, moderate to abundant cytoplasm, distinct cytoplasmic membrane, and no evidence of squamous or glandular differentiation by light microscopy. Some of these tumors may contain features of either squamous and/or glandular differentiation as evidenced by immunohistochemistry or electron microscopy, implying some heterogeneity in this group. Giant cell and clear cell variants are uncommon. A giant cell variant may mimic an anaplastic large cell lymphoma (Ki-1 lymphoma), in that the latter tends to proliferate in lymph node sinuses (similar to metastatic cancer), unlike the usual lymphoma proliferation within the lymph node itself. Clinically, most patients with large cell lung cancer present with bulky, peripheral tumors. Metastases occur early, preferentially to the central nervous system (CNS), and the 5-year survival is under 5%.
Small Cell Lung Cancer
Small cell lung cancer (SCLC) represents about 15% of all lung tumors, is extremely aggressive, is frequently associated with distant metastases, and has the poorest prognosis of all lung neoplasms. The incidence is decreasing for unknown specific reasons; however, it may be related to the greater use of cigarette smokers. SCLC is highly related to cigarette smoking (98% or more of cases). SCLC has a central origin in most cases, although 10% of these tumors are found in the peripheral lung field. The tumors have a white-tan appearance, are friable, and show extensive necrosis. Histologically they are characterized by scant cytoplasm or high nuclear-to-cytoplasmic ratio, fine chromatin, and “nuclear molding.” Small cells are characterized as “small blue cell tumor” and must be distinguished from lymphoma, carcinoid tumors, Ewing’s sarcoma, and primitive neuroepithelial tumors (PNET). The rapid growth and scanty cytoplasm of small cell carcinomas make them unusually susceptible to ischemic necrosis, as well as crush artefact, during handling and fixation. Although not pathognomonic, the so-called Azzopardi effect (crushed DNA material encrusted around blood vessels) is very characteristic ( Fig. 5.21B ). The subclassification of small cell carcinoma into oat cell, intermediate cell, and combined oat cell carcinoma has been dropped from the new WHO classification, and the only subtype of SCLC is combined SCLC. Less than 10% of SCLCs are admixed with non–small cell lung carcinoma (NSCLC) components (with large cells 4% to 6%, 1% to 3% with adenocarcinoma or squamous cell carcinoma).
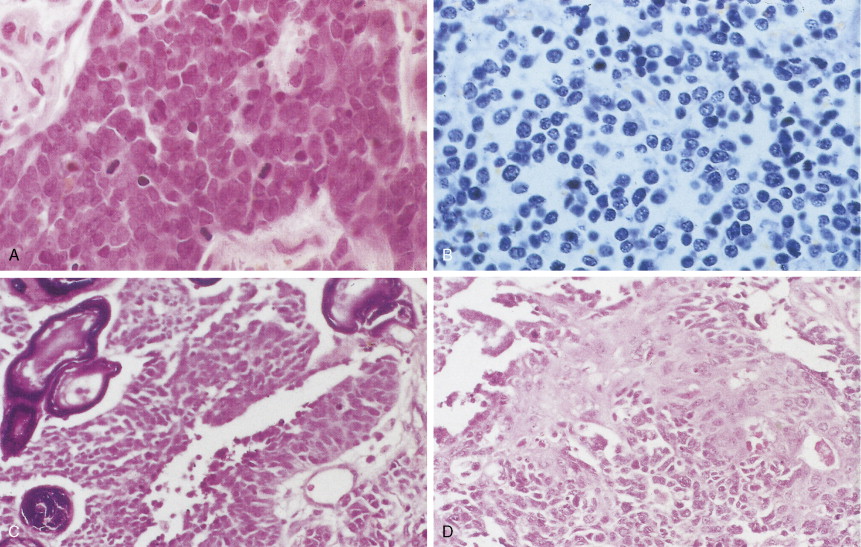
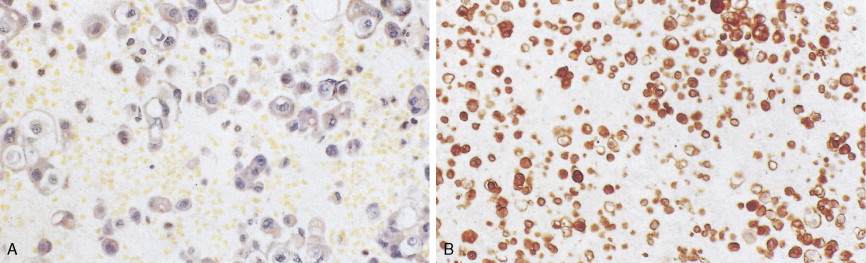
Most SCLCs contain dense-core granules (which contain among other molecules, amines, peptide products, and l -dopa decarboxylase), indicating neuroendocrine differentiation. Immunohistochemical studies demonstrate the presence of neuron-specific enolase (NSE), chromogranin A, Leu-7 (a natural killer cell antigen also present in some neuroendocrine cells), and synaptophysin. Other antigens that may also be expressed are carcinoembryonic antigen (CEA), adrenocorticotrophic hormone (ACTH), and “big” ACTH. “Big” ACTH is a physiologically inactive form of ACTH produced by certain tumors as a paraneoplastic product that is larger and more acidic than “little” (normal) ACTH but immunochemically indistinguishable.SCLC cells, as with most carcinomas, express keratin proteins.
SCLCs produce and release into the circulation a variety of functioning polypeptide hormones that can cause paraneoplastic syndromes ( Table 5.8 ). They also grow in a submucosal pattern with a high frequency of lymphatic and vascular invasion; for this reason they do not often cause hemoptysis. Thus, they may not be readily identified on bronchoscopy. Prominent mediastinal adenopathy is often present. Almost 70% of patients have metastatic disease at the time of diagnosis. Almost any organ can be involved, but preferential sites include the liver, bone, bone marrow, CNS, adrenal glands, abdominal lymph nodes, pancreas, skin, and endocrine organs.
| Type of Manifestation | Frequency (%) |
|---|---|
| Systemic | |
| Anorexia–cachexia | 31 |
| Fever | 21 |
| Suppressed immunity | – |
| Skeletal | |
| Digital clubbing | 29 |
| Periostitis (hypertrophic pulmonary osteoarthropathy)—commonly associated with adenocarcinoma | 1–10 |
| Endocrine | |
| Hypercalcemia (ectopic parathyroid hormone)— commonly associated with squamous cell carcinoma | |
| Hyponatremia (inappropriate secretion of antidiuretic hormone)—commonly associated with small cell carcinoma | |
| Cushing syndrome (ectopic corticotrophin secretion) — commonly associated with small cell carcinoma | |
| Hematologic | 8 |
| Anemia | |
| Granulocytosis | |
| Eosinophilia | |
| Leukoerythroblastosis | |
| Coagulation—thrombotic | 1–4 |
| Venous thrombosis (migratory thrombophlebitis, Trousseau’s syndrome) | |
| Arterial embolism (nonbacterial thrombotic endocarditis) | |
| Hemorrhage (disseminated intravascular coagulation) | |
| Neurologic—myopathic | 1 |
| Eaton-Lambert syndrome (myasthenia)—commonly associated with small cell carcinoma | |
| Peripheral neuropathy | |
| Subacute cerebellar degeneration | |
| Cortical degeneration | |
| Polymyositis | |
| Neurologic—cutaneous | 1 |
| Dermatomyositis | |
| Acanthosis nigricans | |
| Renal | <1 |
| Nephrotic syndrome | |
| Glomerulonephritis |
Carcinoid Tumor and the Spectrum of Neuroendocrine Tumors of the Lung
The classification of neuroendocrine neoplasms of the lung has evolved substantially over the past two decades. Initially there were only two categories: carcinoid and SCLC. The latter is discussed above.
Typical carcinoid or carcinoid tumors (bronchial carcinoid tumors) are similar to tumors arising in the gastrointestinal tract and elsewhere. They are characterized by small (0.7–3.5 cm), well-circumscribed solid tan/yellow nodules with no necrosis or hemorrhage. Usually they are centrally seen, less commonly peripheral in location. By light microscopy tumor cells are round and uniform in size, with finely granular eosinophilic cytoplasm. The nucleus is centrally placed with finely granular or stippled chromatin and small nucleoli. The cells arrange themselves in an organoid pattern (cords, nests, and acini may be formed). Mitoses are rare and necrosis is not seen. By electron microscopy numerous cytoplasmic membrane-bound, dense-core granules (90–450 nm) are usually seen. By immunohistology they are usually positive for NSE, chromogranin A, Leu-7, synaptophysin, bombesin, CEA, ACTH, calcitonin, and keratin. Carcinoid tumors may be responsible for ectopic hormone secretion, particularly 5-hydroxytryptamine, ACTH, vasopressin, and insulin. Typical carcinoid tumors have low malignant potential. They are not usually associated with cigarette smoking.
Atypical carcinoid is a third category described in 1972. Atypical carcinoid is similar to typical carcinoid but usually larger (1.5–2.3 cm) and contains foci of necrosis and mitoses (usually 3–4/10 high-power fields [HPF]). Atypical carcinoids can follow a more aggressive clinical course than typical carcinoid and have metastatic potential. Atypical carcinoids represent approximately 10% of all carcinoid tumors.
The fourth category is large cell neuroendocrine carcinoma (LCNEC). LCNEC is a malignant neuroendocrine neoplasm composed of large polygonal cells with a relatively low nuclear-to-cytoplasmic ratio, coarse nuclear chromatin, frequent nucleoli, high mitotic rate (>10/10 HPF), and frequent necrosis. The cells in LCNEC are larger than cells in SCLC and have more abundant eosinophilic cytoplasm. However, the biology and prognosis of both of these neuroendocrine malignancies is poor as a result of the metastatic disease that occurs early in the natural history and may be refractory to curative treatment.
CHROMOSOMES, GENES, AND LUNG CANCER
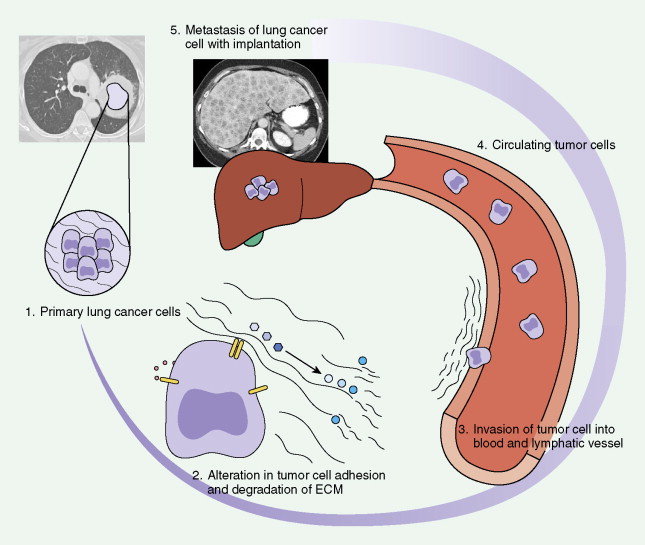
The evolution to cancer in general is currently understood as a multistep process. Insight into this evolution has been gained through recent advances in cytogenetics, cell biology, and mainly molecular biology. It has become apparent that mutations in a limited number of genes, which control cell proliferation and differentiation, are key events in this process. Proto-oncogenes (“activated” by a particular mutation) and tumor suppressor genes (“deactivation” unleashes unregulated proliferation of cells) have major roles in malignant transformation of cells and may be prognostic forms (see Tables 5.5 and 5.6 ). Diagrams for molecular and biochemical abnormalities in lung cancer and mechanisms for metastatic spread are given in Figures 5.1 and 5.2 .
| Variable | Favorable | Unfavorable |
|---|---|---|
| Histopathologic Markers | ||
| 1. Tumor status | T1 | T2 |
| 2. Histologic subtype | Squamous | Large cell † |
| 3. Degree of tumor differentiation | WD | PD ‡ |
| 4. Lymphatic and/or blood vessel invasion | Absent | Present |
| 5. Mitotic index | Low | High |
| 6. Plasma cell infiltration | Present | Absent or minimal |
| 7. Tumor giant cells | Absent | Present |
| 8. WHO subtype of adenocarcinoma | Bronchoalveolar or acinar or papillary | Solid tumor with mucus formation |
| Molecular Genetic Markers | ||
| 1. KRAS oncogene activation | No point mutation | Point mutation at codon 12 |
| 2. RAS gene protein product expression | Absent p21 staining | Strong p21 staining § |
| 3. C- erb -2 protein expression | Normal | Increased |
| 4. TP53 tumor suppressor gene | No mutation | Gene mutation present |
| 5. p53 protein product expression | Normal p53 | Overexpression of p53 |
| 6. Retinoblastoma (RB) protein expression | RB-positive | RB-negative |
| 7. BCL2 protein expression | BCL2-positive | BCL2-negative |
| Differentiation Markers | ||
| 1. Expression of blood group antigen on tumor cells | Conserved expression of blood group antigens | Altered expression of blood group antigens |
| 2. Expression of H/Ley/Leb antigens | Negative staining with MIA-15-5 | Positive staining with MIA-15-5 |
| Proliferation Markers | ||
| 1. DNA content (flow cytometry) | Diploid | Aneuploid |
| 2. S-phase fraction (flow cytometry) | Low | High |
| 3. Mitotic index | <13 mitoses per 10 HPF | ≥13 mitoses per 10 HPF |
| 4. Proliferation index (PI) using Ki-67 nuclear antigen | <3.5 | >3.5 |
| 5. Thymidine labeling index (TLI) | <2.9 | >2.9 |
| 6. Number of nucleolar organizing regions | Mean <3.80/cell | Mean >3.80/cell |
| 7. Proliferating cell nuclear antigen staining | <5% of tumor cells stained | >5% of tumor cells stained |
| Markers of Metastatic Propensity in Stage I NSCLC | ||
| 1. Intensity of angiogenesis | Low microvessel count and density grade | High microvessel |
| 2. Basement membrane deposition (squamous cell carcinoma) | Extensive deposition | Limited deposition ∥︀ |
| 3. Ability to establish in vitro cell lines | In vitro cell lines not established | Independent cell lines |
| 4. Soluble interleukin-2 receptor | Postoperative value less than preoperative | Postoperative value greater than preoperative |
† Adenocarcinoma is intermediate prognosis.
‡ Moderately differentiated is intermediate prognosis.
§ Moderate staining is intermediate prognosis.
∥︀ Moderate deposition is intermediate prognosis.
* Additional poor prognostic factors have been recently identified, including location of lung cancer in the non–upper lobes ( ), visceral pleural invasion (Shimizue et al., 2005), circulating c-MET messenger RNA ( ), loss of expressin of p16 gene ( ), postoperative CEA level >5 ( ). In addition, improved methodology in lung cancer genomic profiling has led to prognostic information that may be useful for adjuvant chemotherapy in patients at high risk of relapse ( .
| Type | Subtype | Cytogenic Abnormalities | Proto-Oncogenes | Onco-suppressor Genes |
|---|---|---|---|---|
| NSCLC | Not specified | 1p13, 3p13 | c- MYC (10%) | TP 53 (50%) |
| 8p11–q11 | BCL -2 | |||
| 8p11–q11 | ||||
| 15p11–q11 | ||||
| 17p11 | ||||
| Chrs. 7, 13, 19 | ||||
| Squamous cell carcinoma | Chr. 11 | erb B-1 | TP 53 (67%) | |
| 3p17q | c- erb B-2 | |||
| c- FOS | ||||
| c- JUN (AP-1) | ||||
| Adenocarcinoma | 3p21.3 (<50%) | KRAS (30%) | TP 53 (37%) | |
| 3p14.1–12.1 | c- erb B-2 (25%) | |||
| c- FOS | ||||
| c- JUN (AP-1) | ||||
| Small cell carcinoma | 3p21.3–3p25 (90%) | c- RAF1 | ||
| 3p14 | ||||
| 5q21 (APC) | c- FMS | TP 53 (80%) | ||
| 6q24 | c- MYB | |||
| 8q24 | c- MYC | |||
| 1p32 | L- MYC | |||
| 13q14 | RB (90%) | |||
| 17q13 |
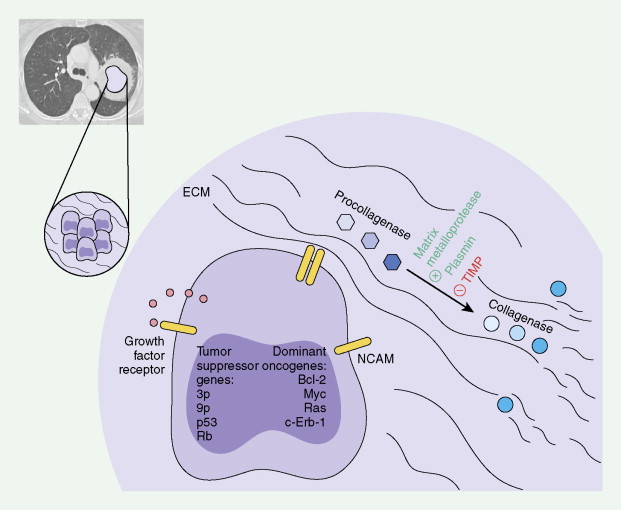
Chromosomal Abnormalities and Telomerase Activation
Using both actual tumor specimens and cell lines, various chromosomal and oncogene abnormalities have been identified. In NSCLC chromosomal aberrations have been described on 3p, 8p, 9p, 11p, 15p, and 17p with deletions of chromosomes 7, 11, 13, or 19. Also, in SCLC, chromosomal abnormalities have been described on 1p, 3p, 5q, 6q, 8q, 13q, or 17p. One of the most consistent chromosomal abnormalities in lung cancer has been the loss of the short arm of chromosome 3 (3p14–p25). The loss of alleles at 3p is observed in more than 90% of SCLC tumors and approximately 50% of NSCLC tumors. As many as three tumor suppressor genes may contribute to SCLC pathogenesis.
Other genetic losses have, though not consistently, been identified in lung cancer. In NSCLC these include genetic loss at chromosome 8p (21.3–p22) and may be abnormal in 50% of tumor samples. Genetic loss at 9p (21–p22) could potentially involve the p16 (MTS1/p16INK4A) and p15 (MTS2/p15INK4B) tumor suppressor genes, which are involved in cell cycle regulation at the G 1 checkpoint by inhibiting cyclin-dependent kinase CDK4 and may be affected in 67% of tumor samples. Genetic loss at 11p (p13 and p15) may involve the Wilms’ tumor suppressor gene at region p13 and can be affected in 20% to 46% of tumor samples.
Telomeres, which are genetic elements at the ends of linear eukaryotic chromosomes consisting of tandem repeats of simple DNA sequences, are important in stabilizing chromosomes from degradation, illegitimate recombination, or cellular senescence. Longer telomeres are present in germ cells and in most cancer cells, via the telomerase enzyme, and these maintain the ability of the cells to divide indefinitely. Telomerase activity has been directly correlated with malignant and metastatic phenotype of a wide array of solid tumors. In one study 80% of tumor tissue from lung cancer had telomerase activity ( ).
Proto-Oncogenes in NSCLC
Amplification of the RAS genes ( KRAS , HRAS , and NRAS ), especially KRAS , is frequent in NSCLC. The level and frequency vary with the tumor type, ranging from about 30% in adenocarcinoma as compared with 10% in other cell types. There is evidence of linking mutations in the RAS family with a poor prognosis in patients undergoing surgery. There is a correlation between KRAS mutations in adenocarcinomas and smoking history. In particular, mutations of the KRAS oncogene involving codon 12 may be a specific target of tobacco smoke and may occur early and irreversibly during carcinogenesis in adenocarcinomas of the lung ( ). Amplification of c- MYC is found in about 10% of NSCLC of all types.
The erb B-1 (epidermal growth factor receptor, EGFR) protein is overexpressed in approximately 60% to 80% of NSCLC, with high expression in squamous cell carcinomas. The EGFR can be mutated somatically in certain subsets of NSCLC (especially adenocarcinomas). The frequency of mutations is highest in the tyrosine kinase domain, nonsmokers, females, and certain ethnic populations (such as Asians). There is also a truncation mutation, EGFR-vIII, that can occur in squamous cell carcinomas. EGFR overexpression can be a prognostication for lung cancer, and the mutations can be a predictive marker for therapeutic response to small molecule tyrosine kinase inhibitors. In certain subsets of NSCLC, EGFR can also be amplified.
The c- erb B-2 (HER2/neu) gene encodes a transmembrane tyrosine-specific protein kinase, p185neu. Frequency of abnormal expression of c- erb B-2 is approximately 25%. This gene is frequently amplified in adenocarcinomas and squamous cell carcinomas; in adenocarcinomas p185neu expression tends to be found in older patients and is usually associated with shorter survival.
The c-MET gene can also be overexpressed in lung cancer. In particular subsets of patients there are also activating mutations and/or amplification that will have prognostic and predictive biomarker implications.
Tumor Suppressor Genes in NSCLC
The TP53 gene encodes a 53-kDa nuclear phosphoprotein identified as a transcriptional activator. High levels of the wild-type gene product inhibit growth, possibly by acting as a checkpoint for DNA damage at the G 0 –G 1 transition in cell division. Mutations in TP53 are very common features in different types of cancer and are present in NSCLC and have been detected in preinvasive lesions of the bronchus ( ). The frequency of mutations varies with the type of NSCLC: about 67% of squamous cell carcinomas and 37% of adenocarcinomas contain TP53 mutations. Mutations are also present in undifferentiated large cell carcinomas. No significant correlation has been found between TP53 mutations and age, sex, histopathology, clinical stage, or lymph node involvement. G:C-to-T:A transversions, found in about 50% of NSCLC, are remarkably uncommon in other types of human cancer. Because one of the components of cigarette smoking is benzo[ a ]pyrene, a potent mutagen that causes G:C-to-T:A transversions, the implication is that smoking may be responsible for these mutations. Interestingly, mutations in both TP53 and KRAS are most commonly G-to-T transversions in lung cancer versus G-to-A transitions in other cancers ( ).
The retinoblastoma gene ( RB1 ) encodes a DNA-binding protein of 110 kDa that is involved in important events of cell division. Inactivation of this gene by deletion and loss of heterozygosity has been found in several cancers, with abnormalities in NSCLC approximately 15%. In NSCLC, an inverse correlation exists between p16INK4A expression and RB1 expression, thereby implicating a key role of these proteins in growth suppression.
Proto-Oncogenes In SCLC
Mutations in RAS genes are absent in SCLC. Gene amplification of all three types of MYC genes has been observed in SCLC. Amplification of MYC genes has been observed more frequently in patients who have undergone chemotherapy, but cell lines established from SCLCs before and after chemotherapy did not alter their status of MYC gene copy number. It is unclear whether chemotherapy can actually cause MYC gene amplification. Increased expression of N- MYC gene has been reported to correlate with poor subsequent response to chemotherapy, rapid tumor growth, and short survival times. c-KIT and c-MET receptor tyrosine kinases are also overexpressed in SCLC.
Tumor Suppressor Genes in SCLC
The RB1 gene (chromosome 3q) is absent or aberrant in over 90% of patients. This was the first identification of a recessive oncogene participating in the pathogenesis of lung cancer ( ). RB1 gene protein has important functions in the regulation of growth stages of cell cycle events, by maintaining cells in a quiescent or growth-arrested state.
Mutations of the TP53 gene are present in over 75% of SCLCs and are considered an early event in carcinogenesis. Structural abnormalities have been detected in some cell lines, but in the absence of RB1 messenger RNA and p105 RB protein.
Chromosomal abnormalities in SCLC mainly consist of chromosome 3 short-arm deletions, in three different regions between 3p21 and 3p25, occurring in over 90% of cases.
Growth Factor Abnormalities in SCLC
In SCLC many of the tumor cells produce neuroendocrine peptides, such as gastrin-releasing peptide (GRP), and respond to them in an autocrine or paracrine fashion. GRP binds to the receptor (G-protein family member) and transduces intracellular signal with proliferation of SCLC cells. Another growth factor, insulin-like growth factor I, is elevated in more than 95% of SCLCs and modulates mitogenic signaling. Also, Steel factor, the ligand for the proto-oncogene tyrosine kinase receptor c-KIT, supports growth and survival of immature hematopoietic cells of multiple lineages. In SCLC, c-KIT and Steel factor are simultaneously expressed, thus forming an autocrine loop.
Metastatic Mechanisms in Lung Cancer
Paget initially observed that metastasis of tumor cells occurred when certain tumor cells (“seed”) had special affinity for the growth environment provided by certain specific organs (“soil”). Tumor cells are heterogeneous and have different angiogenic, invasive, and metastatic properties. Inducing angiogenesis may be an important mechanism for a tumor cell to proliferate and eventually metastasize. Angiogenesis has been shown to be a prognostic factor in stage I NSCLC ( ). Tumor cells can also penetrate pre-existing vessels, thereby leading to metastasis. In one study 15% of patients with tumor invasion of peripheral, node-negative NSCLC had a poor survival rate and a higher recurrence rate ( ).
Angiogenesis is an important part for tumor growth and metastasis. Neoangiogenesis appears to be a significant prognostic factor in patients with resected NSCLC in most, but not all, clinical studies. As an example, one study of tumors from 275 patients with stage I NSCLC analyzed overall survival via multivariate analysis of angiogenesis, proto-oncogene HER2/neu , tumor suppressor gene TP53 , and the proliferation marker Ki-67 (see “HER-2/neu (c-erbB-2) oncogene and protein expression” above and see “TP53 tumor suppressor gene” above). Of these factors, excessive angiogenesis was the most significant adverse prognostic factor. Others have reported a correlation between MVD, PD-ECGF, and VEGF expression, neovascularity, and prognosis in resected NSCLC.
Staging of Lung Cancer
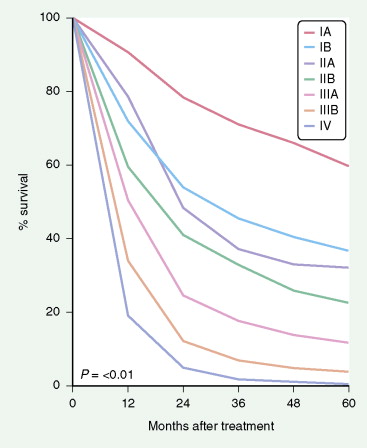
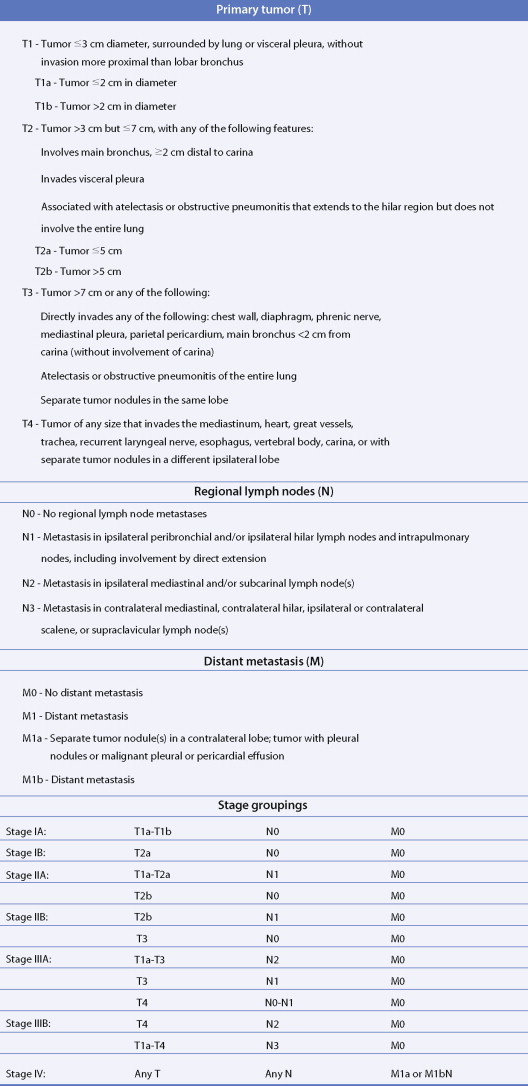
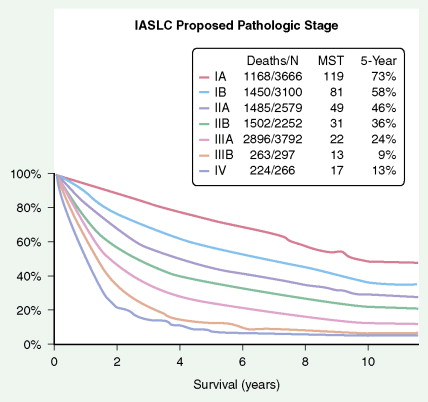
The most widely used system is the International Staging System (ISS) using TNM, categories to place patients into stages I–IV, each having a progressively lower survival rate ( Fig. 5.34 ). It was revised in 1997 with additional stage subgroupings ( ; see also Figs. 5.36 and 5.37 ). Only 30% of patients present with stage I or II disease; 15% to 20% have potentially resectable stage IIIA disease and the remainder have advanced unresectable stage IIIB or metastatic stage IV disease. Although the ISS can be applied to all cell types, SCLC is often categorized as limited disease (stage I, II, or III) or extensive disease (stage IV) for therapeutic purposes. New changes in the TNM classification were first proposed by the International Association for the Study of Lung Cancer (IASCL) at the international meeting in Seoul, Korea, in August 2007 and also in San Francisco, U.S.A., at the thirteenth annual World Conference on Lung Cancer in August 2009. The changes have been accepted by the American Joint Committe on Cancer (AJCC) and the International Union Against Cancer (UICC) and are summarized in Figures 5.31 and 5.32 , along with the survival in a large number of patients according to the pathologic stage ( Fig. 5.33 ). The latter should be compared to survival based on clinical stage ( Fig. 5.34 ) based upon preoperative studies ( ). Survival for patients with SCLC seems to have improved over the last 15 years. However, 2- to 3-year survival still occurs in only 10% to 25% of patients with limited disease and 1% to 2% of patients with extensive disease. Moreover, relapse of SCLC and development of other neoplasms are common in patients surviving beyond 2 years. Prognostic indicators (disease stage) should help target individual SCLC patients for specific intensive treatments designed to prolong survival and achieve cure ( ).
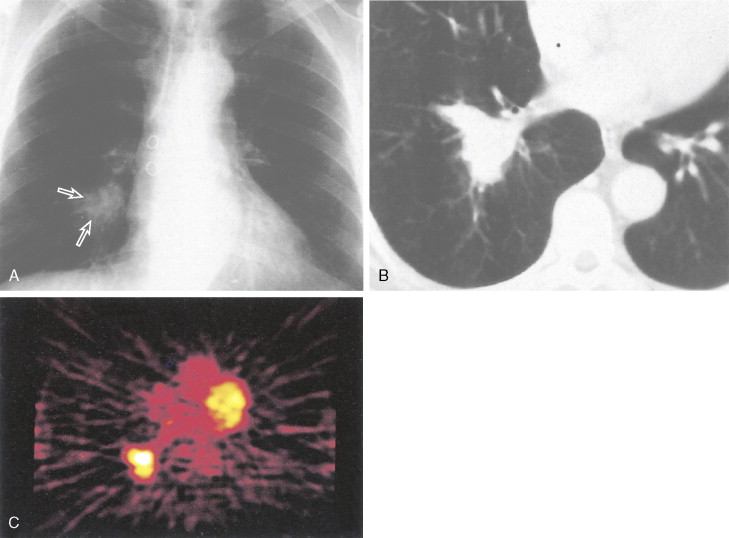

Staging procedures consist of computed tomography (CT) scan of the chest and upper abdomen to include liver and adrenals. Assays for tumor markers (e.g., CEA, CA 125, and NSE; ), if elevated, may be of prognostic value and also allow for monitoring of disease status. Several studies are ongoing to determine the role of molecular markers. A bone scan and head CT scan with contrast magnetic resonance imaging (MRI) should be performed in all patients except for those with stage I NSCLC who are asymptomatic with normal chemistries. In these patients the likelihood of early (occult) metastases is under 5%. Positron-emission tomography (PET) scans are also of value in initial assessment and follow-up restaging or search for metastases (see Chapter 2 ). Whole-body PET using [ 18 F]fluorodeoxyglucose as a tracer is a new imaging technique based upon the increased metabolism of glucose in malignant cells. PET has a 95% sensitivity for detecting primary lung cancers and mediastinal lymph node involvement ( ). The threshold of detection is around 3–5 mm. It may more accurately predict the likelihood of long-term survival than chest CT does ( ). It is also useful to differentiate benign from malignant pulmonary nodules, assess response to treatment and recurrence, and assist in radiotherapeutic planning ( ). Bone marrow involvement as the only stage IV manifestation is unusual, however, and occurs in approximately 5% of cases with limited thoracic disease in SCLC.
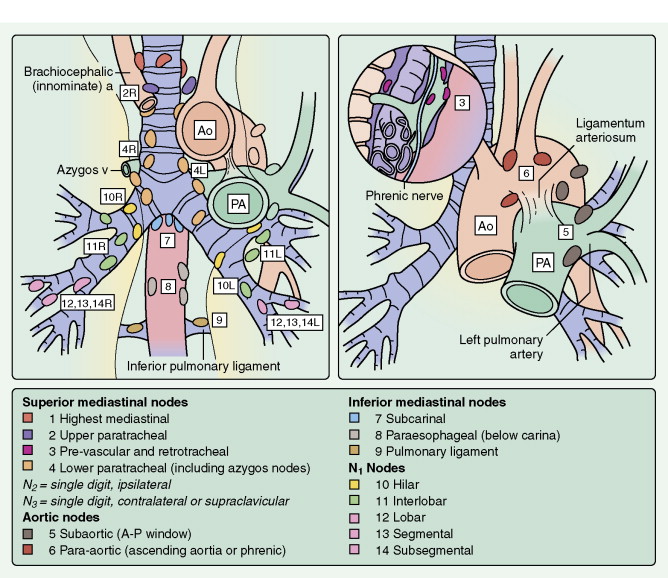
Invasive staging procedures include thoracoscopy, cervical (suprasternal) mediastinoscopy, and anterior mediastinoscopy (Chamberlain procedure). One or more may be carried out to evaluate mediastinal nodal stations (see Fig. 5.30 ) or suspicious sites of disease in resectable patients, particularly when multimodality treatment protocols are utilized. Video-assisted thoracoscopic surgery (VATS) is being used for staging as well as management in selected cases (see Fig. 5.56 ). VATS has minimal mortality and greatly reduces hospitalization time compared with traditional thoracotomy ( ).
CLINICAL MANIFESTATIONS
The signs and symptoms of lung cancer are related directly to the primary malignancy or to distant metastases. Indirect signs and symptoms may be encountered as a result of the secretion of biologically active polypeptides and hormones.
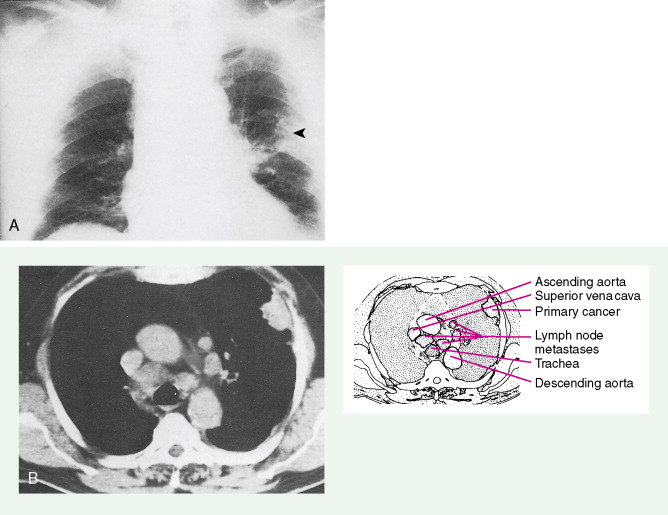
Manifestations of early thoracic disease depend on the location of the primary cancer. Central (proximal) lesions such as squamous cell carcinoma often erode the bronchus, causing hemoptysis and cough. Chest pain is a common symptom in early-stage lung cancer. As the tumor spreads, bronchial obstruction with atelectasis and pneumonia often occurs. Hilar adenopathy and cavitation of the primary cancer may also develop. Although small cell cancers are central in origin, they grow submucosally and thus rarely cause hemoptysis. Due to lymphatic invasion, mediastinal adenopathy occurs in most cases. Extension into the recurrent laryngeal nerve results in hoarseness, and involvement of the phrenic nerve causes a paralyzed (elevated) diaphragm. Stridor, caused by invasion of the trachea or bilateral vocal cord paralysis, results from compromise of the lumen of the trachea. Invasion and compression of the superior vena cava leads to the superior vena cava (SVC) syndrome (see Fig. 5.62 ); this can occur either with isolated stage IIIB disease or as part of stage IV (metastatic) disease. Extension of malignancy into the pericardium results in pericardial effusion and acute cardiac tamponade.