Lung Cancer
 EPIDEMIOLOGY
EPIDEMIOLOGY
Throughout the world, lung cancer accounts for 13% (1.6 million) of the total cases of cancer and 18% (1.4 million) of the cancer-related deaths based on 2008 estimates.1 Among males, lung cancer is the most commonly diagnosed cancer and leading cause of cancer death. Among females worldwide, it is the fourth most commonly diagnosed cancer and the second leading cause of cancer death.
In the United States, lung cancer is the second most common cancer and the most common cause of cancer-related death in both men and women. The American Cancer Society estimates 156,940 people in the United States died of lung cancer in 2011, including 85,600 men and 71,340 women.2 More people in the United States die of lung cancer than from breast, prostate, and colorectal cancer combined. The overall 5-year survival rate for lung cancer is approximately 16%.3
The overall incidence and mortality rate for lung cancer rose steadily from the 1930s until peaking in the early 1990s. The incidence and mortality rates for men began to drop around 1990, and the latest analysis demonstrates a drop in the incidence and mortality rates for women for the first time.4 The lag in the trend of lung cancer rates in women compared with men reflects historical differences in cigarette smoking between the sexes; cigarette smoking in women peaked about 20 years later than in men. Gender and racial disparities exist in the incidence and mortality for lung cancer with rates highest in men, particularly those who are African American.2,5 In terms of socioeconomics, lung cancer demonstrates the largest disparity of all cancers, with the death rate in men five times higher for the least educated than for the most educated.2
Although the lung cancer numbers in the general population are startling, the main risk of lung cancer is based on exposures to carcinogens; most lung cancer cases are attributable to cigarette smoking. Voluntary or involuntary cigarette exposure accounts for 80% to 90% of all cases of lung cancer.6 Since the 1964 landmark report by U.S. Surgeon General citing smoking as a causal agent for the development of lung cancer,7 the prevalence of smoking in the United States has declined significantly. More recently, exposure to secondhand smoke has been considered a risk for lung cancer with up to a 30% increase in risk from secondhand smoke exposure associated with living with a smoker.8 Indoor radon exposure is now considered the second leading cause of lung cancer in the United States.9 Other known risk factors for lung cancer include exposure to occupational and environmental carcinogens such as asbestos, arsenic, and polycyclic aromatic hydrocarbons.10–13
 ANATOMY
ANATOMY
In the past, a simplified and relatively superficial understanding of thoracic anatomy provided an acceptable framework for the radiation oncologist to design treatment fields in lung cancer patients utilizing conventional techniques with the carina and bony structures as landmarks. As the field of thoracic radiation oncology has moved toward more conformal therapy, however, a more detailed understanding of thoracic anatomy is essential for proper target delineation and treatment design.
The lungs are situated on each side of the mediastinum, which contains the heart, trachea, esophagus, and great vessels. The lungs are conical in shape with an apex projecting upward into the neck for approximately 2 to 3 cm above the clavicle, a base sitting on the diaphragm, a costal surface along the chest wall, and a mediastinal surface that is molded to the heart and other mediastinal structures. Visceral pleura cover the lungs, and parietal pleura cover the inside of the chest cavity. The lungs are freely suspended but are rooted to the mediastinum by the structures emerging from the hilum.
The lungs are divided into distinct lobes—three lobes to the right lung and two to the left. The right lung is divided into the upper, middle, and lower lobes by the oblique (major) and horizontal (minor) fissures. The oblique fissure runs forward and downward from approximately the level of the fifth thoracic vertebral body to the diaphragm, dividing the lungs into upper and lower lobes. The horizontal fissure separates the right middle from the right upper lobe, fanning out forward and laterally from the hilum. The middle lobe is thus a small, triangular lobe bounded by the horizontal and oblique fissures and actually rests on the diaphragm. The left lung is divided by only the oblique fissure into two lobes—the upper and lower lobes. The lingula, located in the left upper lobe, is homologous to the right middle lobe and also touches the diaphragm.
The bronchopulmonary segment is the functional unit of the lung and is defined by the segmental bronchi. The trachea bifurcates at the carina, which lies at the junction between the manubrium and body of the sternum, into the right and left main bronchi. Each main bronchus divides into lobar bronchi, each supplying a lobe of the lung. Although the lingula is located in the left upper lobe, the lingular bronchus is considered by many a lobar bronchus. Each lobar bronchus divides into smaller bronchi that form the bronchopulmonary segments. These segments are pyramidal in shape, with an apex toward the lung root and a base at the pleural surface. Structures entering each bronchopulmonary segment (i.e., bronchus and artery) tend to lie centrally. Structures leaving the segment (i.e., veins and lymphatics) lie in the periphery of the segment within the connective tissue that separates the segments.14 Segmental bronchi divide into bronchioles, continue to branch, and eventually form the alveoli, where blood-gas exchange occurs.
The main lymphatic drainage for each bronchopulmonary segment follows the vasculature and airways toward the hilum, where it ultimately drains into the mediastinum. However, the rich network of lymphatics within the thorax leads to complex variability in drainage patterns.15
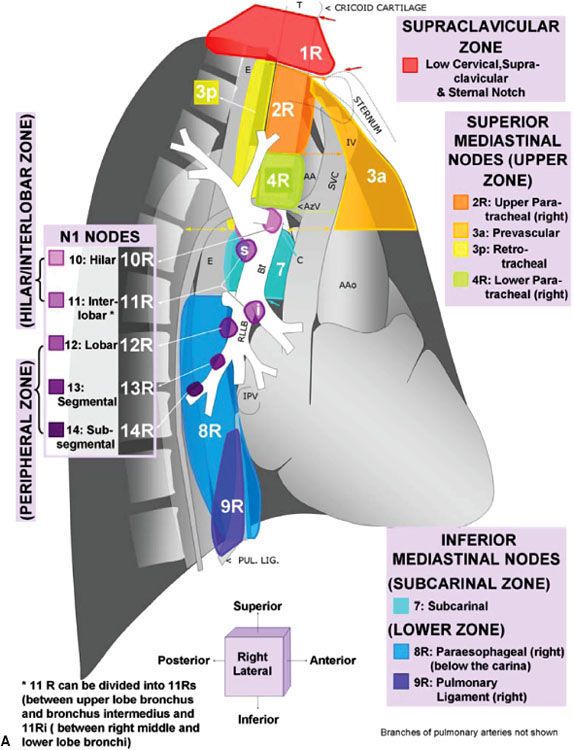
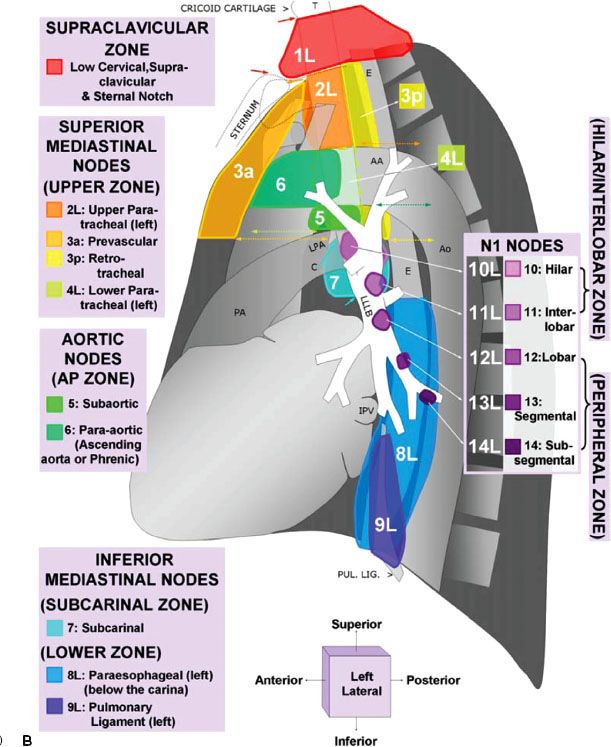
For nearly half a century, lymph node maps have been used in lung cancer to describe the clinical and pathologic extent of lymph node involvement.16 Two such maps—the Naruke lymph node map17 and the Mountain/Dresler map18—have been used the most. Recently, however, the International Association for the Study of Lung Cancer (IASLC) proposed a new lymph node map that reconciles differences among currently used maps and provides precise anatomic definitions for all lymph node stations.16 The IASLC lymph node map has been endorsed by the American Joint Committee on Cancer (AJCC) and incorporated into the seventh edition of its staging manual. Figure 51.1 shows the IASLC lymph node map, which designates 14 levels of intrapulmonary, hilar, and mediastinal lymph nodes stations. The IASLC also compartmentalized the stations into zones that appear to have prognostic implications and are already utilized in common clinical practice.
FIGURE 51.1. The International Association for the Study of Lung Cancer lymph node map for lung cancer staging. (From Lababede O, Meziane M, Rice T. Seventh edition of the Cancer Staging Manual and stage grouping of lung cancer. Chest 2011;139(1):183–189, with permission.)
 CLINICAL PRESENTATION AND PATTERNS OF SPREAD
CLINICAL PRESENTATION AND PATTERNS OF SPREAD
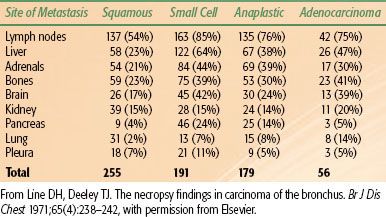
Lung cancer spreads locally by direct extension of the primary tumor, regionally via involvement of the lymphatics, and distantly via invasion into vascular channels leading to hematogenous spread. In a recent Surveillance Epidemiology and End Results (SEER) analysis involving all lung cancer histologies, 15% of all cases of lung cancer were localized to the primary site at initial diagnosis; 22% had regional lymph node spread, and 56% distant metastasis; and the remaining 7% were stage unknown.19 In non–small cell lung cancer (NSCLC), half the patients present with localized or locally advanced disease and half with advanced disease. In small cell lung cancer (SCLC), 20% to 30% present with locally advanced disease, and 70% to 80% present with advanced disease. Table 51.1 shows the site of metastasis based on histologic type.20 Signs and symptoms of lung cancer directly reflect the patient’s local, regional, or distant pattern of spread.
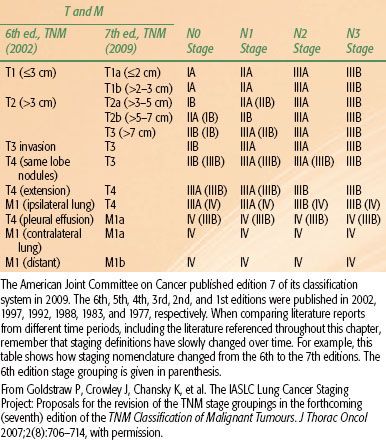
FIGURE 51.2. The American Joint Committee on Cancer released the seventh edition of its lung cancer TNM staging system in 2009. The T classification can be defined by evaluating the size first (upper left), then upgrading the classification (if necessary) based on the other criteria of primary tumor invasion/extent (A, B, and C). The criteria of extent should not be used to assign a lower classification. The lower diagram can be used to define the N and M classification and to determine the corresponding stage. Note that N1, N2, N3, and the separate tumor nodule of M1a were depicted in the lower illustration based on a right-sided tumor (T). For left lung tumors, a mirror image of these descriptors should be used. Additionally, the endobronchial extension and local invasion (A and B of the extent criteria) were shown in the upper illustration based on a left-sided tumor to simplify the drawing. (From Lababede O, Meziane M, Rice T. Seventh edition of the Cancer Staging Manual and stage grouping of lung cancer. Chest 2011;139(1):183–189, with permission.)
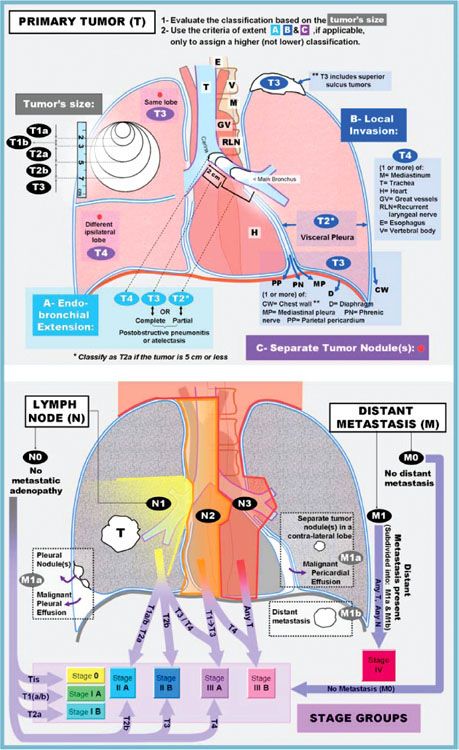
TABLE 51.1 SITE OF METASTASIS CORRELATED WITH HISTOLOGIC SUBTYPE IN LUNG CANCER: NECROPSY FINDINGS IN CARCINOMA OF THE BRONCHUS IN 255 PATIENTS WITH METASTASES TO 431 SITES
Intrathoracic Spread
The intrathoracic spread of lung cancer involves direct extension of the primary tumor or lymphatic spread to regional lymph nodes involving the hilum or mediastinum. There is a wide range of symptoms owing to the intrathoracic effects of lung cancer; the most common include cough, dyspnea, hemoptysis, and chest pain.
The central etiology for many symptoms is owing to a growing tumor involving the airway. Cough is present in 50% to 75% of lung cancer patients at presentation and occurs most frequently in patients with squamous cell and small cell carcinomas because of their tendency to involve central airways.21,22 As central airway involvement progresses, wheezing may develop. Additionally, tumor eroding into a blood vessel or bleeding from the neovasculature supplying the tumor may lead to hemoptysis, which is a presenting symptom in approximately 25% of patients.23 If tumor blocks airflow through a portion of the lung, shortness of breath may develop and is identified at presentation in approximately 25% of cases.21,24 Dyspnea may also be due to the development of atelectasis, postobstructive pneumonia, or a pleural or pericardial effusion.
Chest pain is present in approximately 20% of patients presenting with lung cancer.21,24 Pain may be attributed to direct extension to the mediastinum, parietal pleura, or chest wall. Pleuritic pain may also be the result of obstructive pneumonitis or a pulmonary embolus related to a hypercoagulable state. Pleural involvement can also manifest as pleural thickening or pleural effusion. During the course of lung cancer, 10% to 15% of all cases will eventually develop a malignant pleural effusion.25
Direct extension of a central primary tumor or mediastinal lymph node involvement may lead to nerve involvement. Involvement of the recurrent laryngeal nerve along its course under the arch of the aorta can result in hoarseness. Irritation of the phrenic nerve may initially produce hiccups, and progressive damage can produce unilateral paralysis of the diaphragm with shortness of breath.
Obstruction of the superior vena cava (SVC) from primary tumor or mediastinal lymphadenopathy causes symptoms that commonly include a sensation of fullness in the head and dyspnea. Physical findings include jugular venous distension and occasionally swelling of the face and arms. SVC syndrome is more common in patients with SCLC than NSCLC. The pathophysiology and treatment options for the management of patients with SVC syndrome are discussed in more detail later.
Primary tumors arising within the superior sulcus may produce the classic Pancoast’s syndrome manifested by shoulder pain, Horner’s syndrome, and brachial plexopathy. Pancoast’s syndrome is most commonly caused by NSCLC and only rarely by SCLC. The treatment of patients with superior sulcus tumors (SSTs) will be discussed later.
Distant Extrathoracic Spread
Once vascular or lymphatic invasion occurs, metastatic dissemination to distant sites is common. Contralateral lung, liver, bone, adrenals, and brain are the most frequent sites of distant disease; however, lung cancer can spread to any part of the body (Table 51.1).
Asymptomatic liver metastases may be detected at presentation by liver enzyme abnormalities or on staging workup imaging. Among patients with otherwise resectable NSCLC in the chest, computed tomography (CT) evidence of liver metastasis has been identified in approximately 3% of cases.26 Positron emission tomography (PET) or integrated PET-CT identifies unsuspected metastases in the liver or adrenal glands in about 4% of patients.27,28 Autopsy studies have identified hepatic metastases in >50% of patients with either NSCLC or SCLC.29,30
Pain in the back, chest, or extremity and elevated levels of serum alkaline phosphatase are usually present in patients with bone metastasis. The serum calcium may be elevated owing to extensive bone disease, although the majority of patients with elevated calcium have paraneoplastic parathyroid hormone (PTH)-like syndrome. Approximately 20% of patients with NSCLC and 30% to 40% of patients with SCLC have bone metastases at presentation.31,32 An osteolytic radiographic appearance is more frequent than an osteoblastic one, although a mixed picture is common. The most common sites of involvement are the vertebral bodies.
The adrenal glands are a frequent site of metastasis; however, such metastases are only rarely symptomatic. Concern about adrenal metastasis usually occurs when a unilateral mass is found by staging CT in a patient with a known or suspected lung cancer. Most adrenal masses detected on staging scans are benign, as illustrated by a series of 330 patients with operable NSCLC in which 32 (10%) had an isolated adrenal mass.33 Only 8 of these 32 patients (25%) had a malignancy. Conversely, a negative imaging study does not exclude adrenal metastases. A study of patients with SCLC found that 17% of adrenal biopsies showed metastatic involvement despite having a normal CT scan.34 The lack of specificity of an initial CT scan in identifying an adrenal mass creates a special problem in patients with an otherwise resectable lung cancer. In this situation, PET may be particularly useful in distinguishing a benign from malignant adrenal mass.35 Other procedures that may be useful in excluding a metastasis include magnetic resonance imaging (MRI) consistent with a benign adenoma or a negative needle biopsy. Involvement of the adrenal glands is more frequent in patients with widely disseminated disease. In autopsy series, adrenal metastases were identified in about 40% of patients with lung cancer.30 Patients with an isolated adrenal metastasis but otherwise limited thoracic disease seem to have a much better prognosis than other stage IV disease and may be considered for aggressive definitive management.36,37
Symptoms from brain metastasis include headache, vomiting, visual field loss, hemiparesis, cranial nerve deficit, and seizures. In patients with NSCLC, the frequency of brain metastasis is greatest with adenocarcinoma and least with squamous cell carcinoma. The risk of brain metastasis increases with larger primary tumor size and regional node involvement.38 In patients with SCLC, metastasis to brain is present in approximately 20% to 30% of patients at presentation.39 Without prophylactic irradiation, relapse in the brain occurs in about one-half of patients within 2 years.40 An autopsy series of SCLC patients disclosed central nervous system (CNS) metastases in 80% of cases.41
Paraneoplastic Syndromes
A paraneoplastic syndrome is a disease or symptom that is the consequence of cancer cells in the body but is not attributable to the local presence of tumor. These phenomena are thought to be mediated by humoral factors secreted by tumor cells or by an immune response against the tumor. Treating the cancer, if successful, usually resolves the syndrome. Some of the more common paraneoplastic syndromes are described next.
Cushing Syndrome
Ectopic production of adrenocorticotropic hormone (ACTH) can cause Cushing’s syndrome. Patients typically present with muscle weakness, weight loss, hypertension, hirsutism, and osteoporosis. Hypokalemic alkalosis and hyperglycemia are usually present. Cushing’s syndrome is relatively common in patients with SCLC and with carcinoid tumors of the lung. SCLC patients with Cushing’s syndrome appear to have a worse prognosis than those without Cushing’s syndrome.42–44
Syndrome of Inappropriate Antidiuretic
Hormone Secretion
The syndrome of inappropriate antidiuretic hormone (SIADH) secretion is frequently caused by SCLC and results in hyponatremia. Approximately 10% of patients who have SCLC exhibit SIADH, and SCLC accounts for approximately 75% of all SIADH.45,46 Symptoms include headache, muscle cramps, anorexia, and decreased urine output. If left untreated, cerebral edema can develop, leading to mental status changes, coma, seizures, and respiratory arrest. Besides treating the underlying cancer, demeclocycline is the agent of choice.
Hypercalcemia
Hypercalcemia in patients with lung cancer may be attributable to the secretion of a parathyroid hormone–related protein (PTHrP), calcitriol, or other cytokines, including osteoclast activating factors. In one study of 1,149 consecutive lung cancers, 6% of patients had hypercalcemia.47 Among those with hypercalcemia, squamous cell carcinoma, adenocarcinoma, and SCLC were responsible in 51%, 22%, and 15% of cases, respectively. Symptoms of hypercalcemia include anorexia, nausea, vomiting, constipation, lethargy, polyuria, polydipsia, and dehydration. Renal failure, confusion, and coma are late manifestations.
Lambert-Eaton Myasthenic Syndrome
Lambert-Eaton myasthenic syndrome (LEMS) is an autoimmune disorder characterized by muscle weakness of the limbs that improves with repeated testing, in contrast to myasthenia gravis, which worsens with repetition. Proximal muscles are predominantly affected, and patients complain of difficulty climbing stairs and rising from a sitting position. Approximately 3% of patients with SCLC exhibit LEMS, and SCLC accounts for approximately 60% of all LEMS.48 The neurologic symptoms of LEMS precede the diagnosis of SCLC in >80% of cases, often by months or years.
Hypertrophic Osteoarthropathy
Hypertrophic pulmonary osteoarthropathy (HPO), most frequently associated with adenocarcinoma, is defined by clubbing and periosteal proliferation of the tubular bones. HPO is further characterized by a symmetrical, painful arthropathy that usually involves the ankles, knees, wrists, and elbows. A radiograph of the long bones shows characteristic periosteal new bone formation. A bone scan or PET typically demonstrates diffuse uptake by the long bones. In a series of 111 lung cancer patients, clubbing was present in 29%.49
 SCREENING, DIAGNOSTIC STAGING, AND WORKUP
SCREENING, DIAGNOSTIC STAGING, AND WORKUP
Screening for Lung Cancer
Given the high mortality rate of lung cancer and that the majority of patients are diagnosed at a late stage, lung cancer researchers have theorized that identifying lung cancer at an earlier stage might improve outcomes. Thus, lung cancer has been considered as a candidate for cancer screening. Early screening trials involving chest x-rays and/or sputum failed to demonstrate a survival benefit.50–52 The role of screening has recently been reinvestigated with the advent of spiral CT scans. Early pilot trials of spiral CT in lung cancer screening looked promising with an increase in the identification of stage I detectable cancer. A subsequent international observational trial using spiral CT screening in a cohort of 31,000 high-risk individuals corroborated the findings, showing that annual spiral CT screening could detect lung cancer at an early, potentially more curable stage, suggesting that the stage I disease detection rate and 10-year survival rate could both exceed 80%.53 With a possible mortality benefit theorized but not yet proven, some researchers countered that the use of spiral CT screening might not significantly reduce the mortality risk for lung cancer.54 This set the groundwork for the landmark National Lung Screening Trial (NLST). From August 2002 through April 2004, 53,454 current or former heavy smokers at 33 U.S. medical centers were randomly assigned to three annual screenings with either low-dose CT (26,722 participants) or standard chest x-ray (26,732). Eligible participants were between 55 and 74 years of age with a history of cigarette smoking of at least 30 pack-years and, if former smokers, had quit within the previous 15 years. Persons who had previously received a diagnosis of lung cancer, had undergone chest CT within 18 months before enrollment, had hemoptysis, or had an unexplained weight loss >6.8 kg (15 lb) in the preceding year were excluded. The findings revealed that participants who received low-dose helical CT scans had a 20% relative reduction in risk of death caused by lung cancer compared with the control radiography group (p = .004). Additionally, the all-cause mortality was reduced in the CT screening group by 6.7% when compared with the control group (p = .02). This is the first randomized trial to demonstrate a reduction in all-cause mortality with screening.55 This demonstrates that the benefits to screening outweigh the potential risk from biopsy and further diagnostic intervention in patients who had a false-positive screen. Although this is clearly a landmark study, it is unclear at this time whether this trial alone will lead to a paradigm shift in screening for lung cancer with CT. A key issue relates to the cost-benefit ratio of lung cancer screening with low-dose CT. Several similar randomized CT screening trials are under way.56–60
Diagnostic Staging and Workup
When a patient presents with suspected lung cancer, testing is indicated to confirm the diagnosis, identify the histologic type, and determine the disease stage, all in an effort to guide management decisions. The process begins with a thorough history and physical examination to identify signs or symptoms suggestive of locally extensive or metastatic disease, assess pulmonary health status, identify significant comorbidities, and assess overall health status. Each impacts the therapeutic options in a more comprehensive manner than stage alone. A detailed history should also elicit tobacco use and past exposure to environmental carcinogens. Weight loss >5% from baseline has direct prognostic implications for survival in lung cancer.61–63
Physical examination of the chest may detect signs of partial or complete obstruction of the airways, pneumonia, or pleural effusion. Examination of the neck can reveal evidence of supraclavicular lymphadenopathy. Abdominal examination may detect hepatomegaly. Neurologic examination can detect signs of brain metastasis.
Laboratory studies include complete blood count, liver function tests, and serum electrolytes including calcium. Renal function tests should be performed to assess whether the patient can tolerate intravenous contrast for CT examination or subsequent platinum-based therapy. Liver function test abnormalities could be owing to liver metastasis. Elevation of alkaline phosphatase could be owing to liver or bone metastasis. Calcium elevation could be owing to bone metastasis or paraneoplastic syndrome. Anemia could be owing to metastatic disease.
Radiologic Examinations
Chest X-Ray
Chest x-ray is the initial imaging modality for evaluating a patient with suspected lung cancer. The current x-ray should be compared to prior ones, if available, to determine if a lesion is new, enlarging, or stable.
Computed Tomography
All patients with suspected lung cancer, with or without an abnormal chest x-ray, should undergo a contrast-enhanced CT scan of the chest and upper abdomen to include the entire liver and adrenal glands. Intravenous contrast helps to distinguish vascular structures from mediastinal structures. This not only adds detail to the imaging characteristics of the primary tumor but is also critical to accurately identify suspicious lymph nodes in the mediastinum. CT assessment can establish T-stage by determining tumor size, presence of separate tumor nodules, presence of atelectasis or postobstructive pneumonia, invasion of adjacent structures, and proximal extent of the tumor.
Lymph node enlargement on CT presumes lymph node metastasis in the context of newly diagnosed lung cancer. Most normal mediastinal lymph nodes measure <1 cm, although normal subcarinal lymph nodes can reach a diameter of 1.5 cm. In a patient with known lung cancer, a lymph node is considered suspicious if it measures >1 cm in diameter on its short axis. Unfortunately, many subcentimeter regional lymph nodes may still harbor metastasis. In one study involving pathologic staging, up to 44% of nodes with metastatic deposits were <1 cm in diameter, and 18% of patients with pathologically involved mediastinal nodes did not have any nodes >1 cm.64
Positron Emission Tomography or Positron Emission
Tomography–Computed Tomography
PET scanning has become standard in the staging workup of lung cancer patients. Although the primary tumor characteristics are usually clearly staged with a CT scan, PET can help distinguish atelectasis from tumor in certain cases.65 The largest benefit provided by PET is the identification of suspicious lymph nodes or distant metastasis not seen on CT scan. Kalff et al.66 prospectively evaluated the utility of PET in patients with lung cancer, performing a PET scan on 105 consecutive clinically staged patients with a diagnosis of NSCLC. They found that PET correctly upstaged 26% of patients to palliative from curative intent therapy and appropriately downstaged 10 of 16 patients initially designated for palliative therapy.66 Additionally, PET can detect malignant disease in lymph nodes of normal size, overcoming one of the major limitations of CT.67–69 Integrated PET-CT scanners fuse images obtained in tandem from PET and CT, thus providing both anatomic and metabolic information simultaneously. This is superior to CT or PET alone70,71–72 and can detect malignancy in tumors as small as 0.5 cm.
Although PET has dramatically improved the noninvasive staging of lung cancer patients, it does have some key limitations. On meta-analysis, the sensitivity and specificity of CT for mediastinal nodal metastasis were estimated to be approximately 59% and 79%, respectively, and the sensitivity and specificity of PET were approximately 81% and 90%, respectively.69 This same meta-analysis also found a difference in the accuracy of PET based on the CT size of a lymph node with a sensitivity of 91% for enlarged mediastinal nodes and 75% for nonenlarged nodes.69 Because false positives and false negatives are observed with PET, tissue sampling should be pursued to confirm the presence or absence of regional lymph node involvement before a treatment decision is made. A positive PET should not be considered proof of lymph node metastasis, especially if such a conclusion would otherwise exclude surgery.
With highly conformal radiation therapy for lung cancer becoming commonplace, PET is now being used to aid radiation oncologists in the target delineation process for involved-field radiotherapy (IFRT).73,74 Registration of PET with the planning CT scan at the contouring stage has been shown to enhance the accuracy of defining gross tumor volumes (GTVs).75 The clinician must be mindful that target volumes based solely on 18F-fluorodeoxyglucose (FDG)-PET positivity have their limitations, with the most notable being a false-negative rate of approximately 25% in mediastinal lymph nodes <1 cm in size.69 Additionally, the optimal windowing algorithm for the purposes of contouring remains to be determined. Depending on the algorithm employed, the volume that is contoured can vary significantly.76 When benchmarked against the true pathologic volume, at present the CT-derived contour appears to be more accurate than that derived from FDG-PET regardless of the algorithm employed.77 Efforts are under way to improve on the accuracy of current PET scanning, and one approach is through the exploration of novel tracers. Standard PET scanning focuses on glucose metabolism with FDG as the radionuclide. Other PET tracers currently being explored for interrogation of distinct components of tumor biology include 18F-fluoromisonidazole (FMISO) and 2-(2-nitro-(1)H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)-acetamide (EF5) for tumor hypoxia; 18F-fluorothymidine (FLT) for tumor proliferation; and 11C-methionine and 11C-tyrosine for amino acid metabolism.78,79 In addition to potentially improving the accuracy of target delineation, integrating the functional information from these novel tracers into the treatment planning process provides an opportunity for dose escalation to areas of radioresistance.79
Special Diagnostic Procedures
Sputum Cytology
Sputum cytology is a rapid, relatively inexpensive but underused means to establish a tissue diagnosis in an individual with a suspected pulmonary carcinoma. Previous reports have indicated that the sensitivity of sputum cytology is 65% in the setting of established cancers.80 Three specimens increase the diagnostic yield. Sputum samples are considered representative if alveolar macrophages and bronchial epithelial cells are present. The diagnostic yield of sputum cytology is enhanced with centrally located, intraluminal cancers such as squamous cell carcinoma.
Percutaneous Fine Needle Aspiration
CT-guided fine needle aspiration (FNA) is an excellent method for establishing a tissue diagnosis from a suspicious peripheral pulmonary nodule that cannot be reached by bronchoscopy. The risk of a pneumothorax from this procedure is 25%. However, most of these are small, asymptomatic, and resolve without intervention; only approximately 5% require a chest tube. The overall diagnostic yield is 80%.81 Indeterminate biopsies must be interpreted with caution. FNA cannot rule out malignancy unless another benign diagnosis can clearly be established. Abnormalities involving bone, liver, and adrenal glands can also be confirmed by CT-guided FNA. Frequently, biopsy of one of these suspected metastatic sites simultaneously establishes tissue diagnosis and stage of the disease. Increasingly, as we enter the modern era of molecularly guided therapy, core biopsies are displacing FNAs. This increases the risk but also increases the yield.
Bronchoscopy
Fiberoptic bronchoscopy enables visualization of the tracheobronchial tree to the second or third segmental divisions. Cytologic brushings or biopsy forceps specimens can be obtained from identified lesions. Even when no visible lesion is identified, the bronchus draining the area of suspicion can be lavaged for cytologic analysis. With the use of fiberoptic bronchoscopy combined with special CT imaging techniques, even more peripheral lesions can be reached.82 The diagnostic yield of fiberoptic bronchoscopy is directly related to the ability of the operator to navigate the scope to the lesion of interest; this can be challenging for peripheral lesions. The superDimension/Bronchus system is a real-time guidance system designed to guide the bronchoscope to specified locations within the bronchial tree. Using this approach, a virtual map of the bronchial tree is generated from a high-resolution CT scan of the chest, enabling the physician to monitor the location of the bronchoscope in real time through feedback from a positional sensor attached to the tip of the bronchoscope. There is evidence that this approach may significantly improve the diagnostic yield for peripheral lesions.82
Endoscopic Fine Needle Aspiration
Fiberoptic endoscopy techniques can also be combined with ultrasound to evaluate mediastinal and hilar lymph nodes. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) involves FNA sampling of ultrasound-suspicious lymph nodes, especially those located in the paratracheal (lymph node levels 2 and 4), subcarinal (level 7), or hilar lymph node stations (level 10).83 A prospective study comparing EBUS-TBNA with PET-CT scans revealed an accuracy of 98% and a sensitivity and specificity of 92% and 100%, respectively.84 An esophageal approach known as transesophageal endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) can perform the same function, especially the sampling of mediastinal nodes that are posterior or inferior, such as the retrotracheal (lymph node station 3p), subcarinal (level 7), paraesophageal (level 8), and pulmonary ligament lymph nodes (level 9).85,86
Thoracentesis
Most pleural effusions in lung cancer patients are owing to tumor and should be evaluated with thoracentesis. In general, a diagnosis of cancer can be established in 70% to 80% of malignant effusions by thoracentesis.87 Even if cytology fails to identify cancer cells, repeat thoracenteses improve the diagnostic yield. If on multiple taps the fluid is consistently bloody or exudative, it should be considered malignant. Light’s criteria88 can be used to help classify an effusion as exudative or transudative. As an alternative to repeat thoracentesis, thoracoscopy can be used to simultaneously collect pleural fluid for cytology, visualize the pleural space and biopsy suspicious lesions if present, and perform lymph node biopsies if indicated.
Mediastinoscopy and Mediastinotomy
Mediastinoscopy remains the most accurate technique to assess upper and lower paratracheal (lymph node stations 2 and 4), prevascular (station 3a), retrotracheal (station 3p), subcarinal (station 7), and hilar lymph nodes (station 7) in lung cancer patients. Lymph nodes within the aortopulmonary window (lymph node station 5) and along the ascending aorta (station 6) are not accessible by standard mediastinoscopy techniques; however, they can be evaluated by anterior mediastinotomy (also known as the Chamberlain procedure) or video-assisted thoracoscopic techniques. Although considered the gold standard, mediastinoscopy does have a false-negative rate of approximately 10%.89 Furthermore, the role of mediastinoscopy for lung cancer has evolved recently. Less invasive techniques such as EBUS-TBNA or EUS-FNA are frequently utilized instead to sample lymph nodes found to be clinically suspicious on imaging.90 Mediastinoscopy should still be considered in situations where less invasive techniques are nondiagnostic. It is reasonable to forgo invasive staging of the mediastinum in patients with clinical stage I peripheral disease, particularly those with PET-positive primary tumors but no mediastinal uptake and no obviously enlarged nodes on CT. Patients with more locally advanced disease being considered for surgery should undergo mediastinoscopy to rule out N3 disease and to identify those with N2 disease for whom induction therapy should be considered prior to surgery.
Thoracoscopy
Video-assisted thoracoscopy is frequently used for the diagnosis, staging, and resection of lung cancer. Peripheral nodules can be identified and excised using video-assisted, minimally invasive techniques. As discussed previously, this technique is also extremely valuable for evaluation of suspected pleural disease when thoracentesis has been nondiagnostic. Thoracoscopy can also be used to reach mediastinal nodes not accessible by standard mediastinoscopy, EBUS-TBNA, or EUS-FNA techniques.
TABLE 51.2 AJCC STAGING OF LUNG CANCERA

TABLE 51.3 STAGE GROUPING: TNM SUBSETS
 STAGING
STAGING
The current seventh edition of the AJCC Cancer Staging Manual represents a major change in the developmental process of the staging system. The IASLC established a Lung Cancer Staging Project in 1998 to bring together larger databases available worldwide. The IASLC lung cancer database is comprised of 81,015 cases available for analysis from 46 sources in more than 19 countries, diagnosed between 1990 and 2000, and treated by all therapeutic modalities.91,92 The results of this project were accepted by the AJCC as the primary source for revisions of the lung cancer staging system in the seventh edition of their staging manual. Definitions of TNM for the seventh edition of the manual are shown in Table 51.2; the stage groupings are shown in Table 51.3. A reference chart can be found in Figure 51.2. This new staging system more accurately expresses the prognostic significance of both the T and N stages in lung cancer outcome.
Given all of the various ways to assess lymph node status, accuracy in determining the nodal stage is essential.93 In accordance with IASLC recommendations adopted by the AJCC, when pathologic staging of lymph nodes is pursued, sampling of paratracheal (stations 2R and 4R), subcarinal (station 7), hilar (station 10R), and interlobar lymph nodes (station 11R) should be obtained for right-sided tumors, and aortopulmonary window (station 5), ascending aorta (station 6), subcarinal (station 7), hilar (station 10L), and interlobar (station 11L) lymph nodes for all left-sided tumors. Pulmonary ligament lymph nodes (station 9) should also be evaluated for lower lobe tumors. At least six lymph nodes (three from the mediastinum and three from the hilar region) should be examined. If all resected lymph nodes are negative but the number recommended is not met, the patient is still classified as pN0 in the AJCC staging system.3 For the radiation oncologist, proper staging of nodal disease has taken on increased importance as advances in conformal radiotherapy have resulted in elective mediastinal nodal irradiation being replaced by IFRT.94
FIGURE 51.3. Patterns of failure. Sites of locoregional recurrence after surgical resection of 26 right upper lobe, 6 right lower lobe, 20 left upper lobe, and 7 left lower lobe tumors. A distinction is made between isolated recurrences (patients with a single recurrent site: open stars) and nonisolated recurrences (filled stars). (From Kelsey CR, Light KL, Marks LB. Patterns of failure after resection of NSCLC. Int J Radiat Oncol Biol Phys 2006;65(4):1097–1105, with permission from Elsevier.)
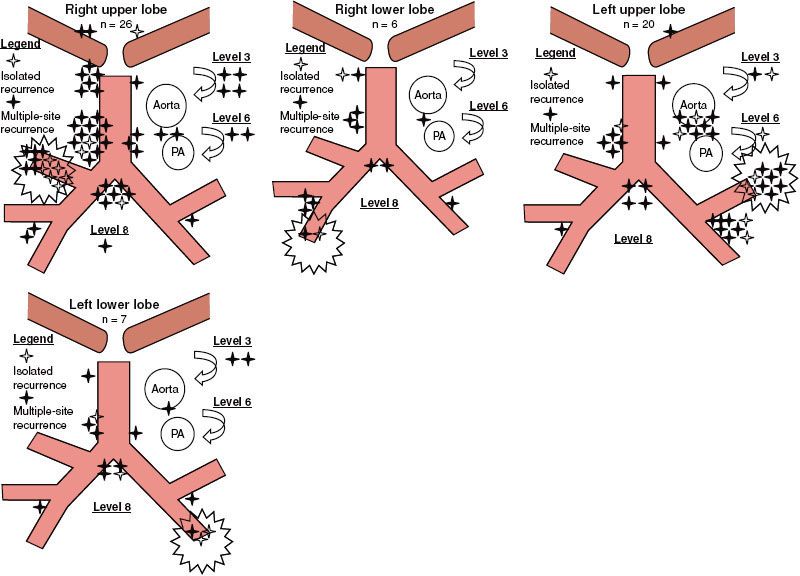
TABLE 51.4 HISTOLOGIC CLASSIFICATION OF LUNG CANCER IN RESECTED SPECIMENS

 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
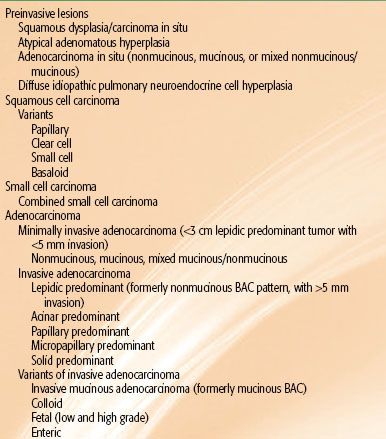
The pathologic classification of lung cancer is undergoing significant transformation, driven primarily by recent therapeutic advancements in the management of advanced disease and the movement toward minimally invasive tissue acquisition procedures. The primary charge of the pathologist in the past had been to distinguish between non–small cell carcinoma and small cell carcinoma of the lung. However, since 2000, there are now important therapeutic implications for each of the four major classifications of lung cancer: (a) squamous cell carcinoma, (b) adenocarcinoma of the lung, (c) small cell carcinoma, and (d) large cell carcinoma. Histology—for the first time—is an important determinant in the selection of systemic therapy for advanced NSCLC. Bevacizumab, a monoclonal antibody targeting VEGF, resulted in grade 4 and 5 pulmonary hemorrhage in patients with squamous cell histology; however, this agent in combination with standard chemotherapy has yielded a statistically significant survival advantage in patients with nonsquamous histology.95,96 An association between nonsquamous histology (including adenocarcinoma and large cell carcinoma) and improved survival has been observed with pemetrexed in combination with platinum-based chemotherapy.97,98 Adenocarcinoma histology is often associated with the presence of epidermal growth factor receptor (EGFR) mutations that confer heightened sensitivity to EGFR tyrosine kinase inhibitor (TKI) therapy and with echinoderm microtubule associated protein like 4 (EML4) and anaplastic lymphoma kinase (ALK) translocations that confer sensitivity to the MET/ALK inhibitor crizotinib.99–102,103 All of these changes are being incorporated into the pathologic classification scheme for lung and have resulted in modifications to the 2004 World Health Organization (WHO) classification scheme for resected specimens104 (Table 51.4). Additional changes that include the classification of specimens obtained from minimally invasive procedures are reviewed extensively elsewhere.105,106
 PROGNOSTIC AND PREDICTIVE FACTORS
PROGNOSTIC AND PREDICTIVE FACTORS
The recent advancements in our understanding of tumor biology have underscored the fact that lung cancer is a heterogeneous cluster of illnesses rather than simply a dual (small cell, non–small cell) disease entity. Given the varied clinical outcomes and significant toxicity of treatment, there is an underlying need for robust prognostic and predictive factors in this disease. Prognostic factors, such as TNM stage, are those that predict clinical outcome independent of therapy, and predictive factors are those that predict response to a particular therapeutic regimen. In general, a discussion of prognostic and predictive factors is divided into two categories: tumor-related factors and patient-related factors. In the near future, these factors may be incorporated into a comprehensive approach to tailor therapy not simply by TNM stage but individualized to the patient and the biology of the patient’s tumor.
Tumor-Related Prognostic and Predictive Factors
The past decade has seen significant advances in our understanding of tumor-related prognostic and predictive factors. Excision repair cross-complementation group 1 (ERCC-1)—a key enzyme involved in the pathway that repairs DNA adducts formed with cisplatin—has been identified to be both a positive prognostic factor for survival (ERCC-1 positivity predicts for improved survival after surgical resection) and a negative predictive factor for response to cisplatin-based chemotherapy (low levels of ERCC-1 predicts for response to cisplatin).107 Activating mutations in the EGFR have been demonstrated to be predictive for response to therapy with EGFR inhibitors, such as gefitinib.108 Thymidylate synthase (TS), which is an important enzyme in DNA biosynthesis and one of the target enzymes for antifolate drugs, has been proposed as a predictive factor for response to therapy in NSCLC patients receiving pemetrexed, a multitargeted antifolate approved for first-line therapy in combination with cisplatin in advanced lung cancer patients with nonsquamous histology. High levels of TS have been shown in vitro and in vivo to correlate with resistance to pemetrexed, and TS gene expression has been shown to be significantly higher in squamous cell carcinomas than in nonsquamous tumors.109–111
Patient-Related Prognostic and Predictive Factors
Given the significant metabolic toll that lung cancer takes on patients, several patient-related factors have been identified as powerful prognostic indicators of clinical outcome. Performance status, as quantified by either the Karnofsky scale or the Eastern Cooperative Oncology Group (ECOG) scale, and weight loss in the 6 months preceding diagnosis have been shown in large trials to be among the factors most predictive of survival (along with TNM stage).112 Additionally, age, gender, and marital status have been shown to be prognostic for survival in a number of studies.113 More recently, Movsas et al.114 reported that baseline quality of life (QOL), as quantified by validated instruments, superseded performance status, age, gender, and other classic prognostic indicators for survival in a prospectively collected data set in patients enrolled on a Radiation Therapy Oncology Group (RTOG) clinical trial, suggesting that QOL may be one of the most important predictors of long-term survival.114
 GENERAL MANAGEMENT: NON–SMALL CELL LUNG CANCER
GENERAL MANAGEMENT: NON–SMALL CELL LUNG CANCER
The management of NSCLC presents a formidable challenge. Oncologists must not only account for the stage and extent of disease spread at the time of diagnosis but also must carefully weigh the impact of baseline pulmonary functional status and comorbidities on the patient’s ability to tolerate treatment. NSCLC is an aggressive tumor of a vital organ that is poorly functioning at baseline in the majority of patients. Therefore, the final therapeutic approach must be tailored to the individual. The treatment principles presented in this section should be viewed as guidelines and not as a cookbook for the management of this disease.
In general, the standard of care for patients with stage I and stage II disease is complete surgical resection with the possible addition of adjuvant chemotherapy. For stage III patients, a significant amount of controversy exists regarding optimal management. For select patients with stage IIIA disease at diagnosis who are candidates for surgical resection, neoadjuvant chemotherapy or chemoradiotherapy is often used. For patients with unresectable stage III disease, the standard approach is concurrent chemoradiotherapy for fit patients or sequential chemotherapy and radiotherapy for patients who cannot tolerate concurrent treatment. Approximately 50% of patients present with evidence of hematogenous dissemination at the time of diagnosis.115 For stage IV patients without significant local presenting symptoms or need for urgent radiation, systemic chemotherapy is the standard initial treatment approach. For stage IV patients with significant local presenting symptoms requiring urgent radiotherapy, such as SVC obstruction, hemoptysis, or cord compression, palliative radiotherapy followed by systemic chemotherapy is the preferred treatment approach. For patients with stage IV disease, owing to the poor prognosis, a detailed discussion of the goals of care with consideration of early referral to hospice should be part of the initial treatment approach. Recent evidence suggests that early introduction of palliative care into the standard treatment paradigm in this setting not only improves QOL and reduces inappropriate hospitalization at the end of life but may also improve survival.116
Resectable Tumors
Preoperative Assessment
Patient selection is critical when an operative approach is being considered for the management of NSCLC. This includes an assessment by the pulmonologist and operating surgeon of the clinical extent of disease, the predicted postresection pulmonary reserve of the patient, and preoperative cardiac clearance for the intended surgical procedure. Although there are no strict guidelines for operability, traditionally patients are considered to be suitable for pneumonectomy if their predicted postoperative forced expiratory volume in 1 second (FEV1) is >1.2 L.117 Additional contraindications to pneumonectomy are hypercarbia and cor pulmonale.118,119 Patients are usually referred for preoperative pulmonary function testing, including spirometry, diffusion capacity, and arterial blood gases. Imaging studies include ventilation-perfusion imaging to determine the regional variance in pulmonary function, including the potential loss of functional lung tissue within the planned area of excision.
Stage I and Stage II Non–Small Cell Lung Cancer
The standard of care for a patient with stage I or II lung cancer is surgical resection through either a lobectomy or pneumonectomy with mediastinal lymph node dissection. The Lung Cancer Study Group performed a randomized trial of lobectomy versus limited surgical resection (either through a wedge resection or through segmentectomy) in patients with T1N0 or T2N0 NSCLC. This trial randomized 276 patients to either lobectomy or limited resection and found a 17% risk of local recurrence with limited resection versus 6% with lobectomy (p = .008). This observation was associated with a trend toward an increase in all-cause and cancer-specific risk of death in patients randomized to the limited resection (30% and 50% increased risk, respectively [p = .09], for both). Additionally, the report did not demonstrate any late functional advantages or decreased perioperative morbidity with limited resection.120 This study firmly established lobectomy as the standard of care for early-stage lung cancer. The 5-year survival with lobectomy or pneumonectomy with mediastinal nodal dissection is approximately 60% for pN0 disease and 40% in pN1 disease.121
In a series from Memorial Sloan-Kettering Cancer Center, Martini et al.122 documented a survival with lobectomy or pneumonectomy with complete mediastinal nodal dissection of 82% in T1N0 tumors at 5 years and 74% at 10 years, and 68% at 5 years and 60% at 10 years for patients with T2N0 tumors (p <.0004). They reported a decreased 5- and 10-year survival rate of 59% and 32%, respectively, in 38 patients who did not undergo lymph node dissection. Additionally, they observed poorer 5- and 10-year survival with wedge resection or segmentectomy of 59% and 35%, respectively, compared with 77% and 70% for patients who underwent lobectomy, corroborating the findings of the Lung Cancer Study Group report. The authors additionally documented second primary cancers in 206 of 598 (34%) patients; 70 of these were lung cancers (34% of second primary cancers). Martini et al.122 concluded that “(1) Systematic lymph node dissection is necessary to ensure that the disease is accurately staged; (2) lesser resections (wedge/segment) result in high recurrence rates and reduced survival regardless of histologic type; and (3) second primary lung cancers are prevalent in long-term survivors.” However, some controversy remains regarding their assertions in the role of mediastinal lymph node dissection in early-stage NSCLC as well as the contention that a sublobar resection is a “compromised” surgical procedure. The American College of Surgeons Oncology Group (ACOSOG) Z0030 trial was a randomized trial of 1,111 patients with N0 or N1 (less than hilar) early-stage NSCLC to either mediastinal lymph node sampling or complete lymphadenectomy during pulmonary resection. The 5-year disease-free survival was 69% in the mediastinal lymph node sampling group and 68% in the mediastinal lymph node dissection group (p = .92). There was no difference in local (p = .52), regional (p = .10), or distant (p = .76) recurrence between the two groups, suggesting that complete lymphadenectomy does not improve survival in patients with early-stage NSCLC.123 There have been several recent series comparing sublobar resection in appropriately selected early-stage NSCLC with lobectomy, showing comparable oncologic outcomes with the more limited resection. Okada et al.124 performed a retrospective multi-institutional comparison of 567 patients undergoing either a sublobar (N = 305) or a lobar (N = 262) resection for cT1N0M0 (tumor size <2 cm) disease. With a median follow-up >5 years, they reported a 5-year overall survival (OS) of 89.6% for the sublobar resection group and 89.1% for the lobar resection group. The recurrence rate with sublobar resection was not inferior to those obtained with lobar resection, and postoperative lung function was significantly better in patients who underwent sublobar resection.124 Two currently active prospective randomized trials are examining the role of sublobar resection in early-stage disease: the Cancer and Leukemia Group B (CALGB) 140503, a randomized trial of sublobar resection versus lobectomy in small, peripheral early-stage operable NSCLC, and ACOSOG Z4032, a prospective randomized trial of sublobar resection with or without brachytherapy for high-risk early-stage NSCLC. These trials will delineate the role of sublobar resection in the management of early-stage NSCLC.
Stage III Non–Small Cell Lung Cancer
Considerable controversy exists regarding the role of surgery in stage III NSCLC. In the 1960s and 1970s, patients with documented N2 disease were generally regarded as incurable and referred for nonoperative approaches. In 1981, Martini et al.125 reported the outcome of 80 patients with documented N2 disease who underwent complete surgical resection and mediastinal lymph node dissection. Most patients also received postoperative mediastinal irradiation. Survival was 47% at 3 years and 38% at 4 years, with better survival associated with adenocarcinoma histology. Additionally, patients who had small primary tumors and nonbulky mediastinal nodes (i.e., no evidence of mediastinal enlargement on preoperative chest x-ray) had better survival. This study suggested a potential role for surgical resection in select patients with N2 disease.125 Martini et al.126 extended this observation in a second report in an expanded cohort of 1,598 patients who underwent surgical resection, 706 of whom had mediastinal nodal involvement. Of these, 151 patients underwent complete surgical resection with mediastinal node dissection. They reported an OS rate of 74% at 1 year, 43% at 3 years, and 29% at 5 years. Survival in patients with clinical stage I or II (pathologic N2) was favorable at 50% at 3 years. Survival in patients with obvious clinical N2 disease was extremely poor at 8% at 3 years. Martini et al.126 stated: “Very few patients with gross mediastinal nodal involvement benefit from resection. We believe that this group of patients should not be considered for thoracotomy unless innovative forms of treatment can be offered.”
Based, in part, on these and similar results with surgical resection alone in stage III disease, neoadjuvant approaches—either preoperative chemotherapy or chemoradiotherapy—were explored in an attempt to facilitate surgical resection. However, significant controversy still exists regarding the role of surgical resection in stage III disease. Van Meerbeeck et al.127 reported the results of a European Organisation for Research and Treatment of Cancer (EORTC) phase III randomized trial of surgical resection versus radiotherapy after induction chemotherapy in patients with pathologically proven N2 disease. In this study, 579 eligible patients were enrolled and received three cycles of cisplatin-based induction chemotherapy. The 332 patients who responded to induction chemotherapy were then randomized to surgery (167 patients) or radiotherapy (165 patients). Median and 5-year OS for patients randomly assigned to resection versus radiotherapy were 16.4 versus 17.5 months and 15.7% versus 14%, respectively (hazard ratio [HR] 1.06, 95% confidence interval [CI] 0.84 to 1.35). Rates of progression-free survival (PFS) were also similar in both groups. The authors concluded that radiotherapy is the preferred approach in these patients owing to lower rates of treatment-related morbidity and mortality.127
TABLE 51.5 SURGERY ALONE VERSUS NEOADJUVANT CHEMOTHERAPY FOLLOWED BY SURGERY IN STAGE III NON–SMALL CELL LUNG CANCER
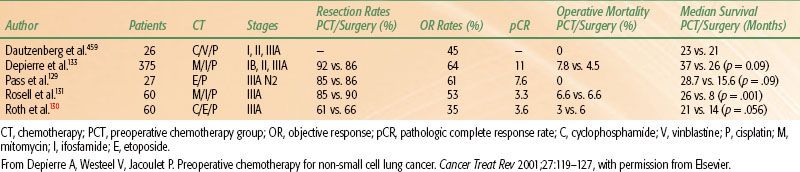
Neoadjuvant (Induction) Therapy
Preoperative Chemotherapy
The primary rationale for induction chemotherapy is similar to the rationale for preoperative radiotherapy: to facilitate complete surgical resection of disease. Additionally, induction chemotherapy may potentially sterilize micrometastatic disease beyond the thorax. In 1988, Martini et al.128 reported on a series of 41 patients with bulky mediastinal nodal involvement, so-called clinical N2 disease, who had evidence of mediastinal nodal enlargement on chest x-ray. Patients received two or three cycles of high-dose cisplatin with vindesine or vinblastine, with or without mitomycin C. Thirty-one (73%) patients had a major radiographic response, 28 patients underwent thoracotomy, and 21 patients (75%) had complete resection of the disease. Eight patients had a pathologic complete response, with an additional 4 patients having “limited microscopic foci of either residual primary or nodal disease.” The 3-year survival was 34% for all patients and 54% for those who had complete resection with a median follow-up of 44 months.128 These promising early results prompted several randomized trials comparing preoperative chemotherapy versus surgical resection alone in stage III NSCLC (Table 51.5). Pass et al.129 reported the results of a small trial randomizing 27 patients with histologically confirmed N2 disease to preoperative etoposide and cisplatin (EP) followed by surgical resection versus immediate surgical resection with postoperative mediastinal radiation. The initial report showed a trend toward increased survival time for the patients who received preoperative chemotherapy (median, 28.7 months) versus the immediate surgery group (median, 15.6 months) (p = .095). Two separate randomized trials were reported in 1994, both of which were terminated early after enrollment of only 60 patients. Roth et al.130 randomized 60 patients with resectable, pathologically confirmed IIIA NSCLC between 1987 and 1993 to receive either six cycles of perioperative chemotherapy (cyclophosphamide, EP) and surgery (28 patients) or immediate surgery alone (32 patients). Patients who had a pathologically confirmed tumor response after three cycles of preoperative chemotherapy received three additional cycles of postoperative chemotherapy for a total of six cycles. Patients randomized to perioperative chemotherapy and surgery had an estimated median survival of 64 months compared with 11 months for patients who had immediate surgical resection (P <.008). The estimated 2- and 3-year survival rates were 60% and 56% for the perioperative chemotherapy patients and 25% and 15% for those who had surgery alone. The trial was terminated early because of the magnitude of the treatment benefit for perioperative chemotherapy after an unplanned interim analysis.130
Similarly, Rosell et al.131 randomized 60 patients with pathologically confirmed IIIA NSCLC to either immediate surgical resection or three cycles of mitomycin C, ifosfamide, and cisplatin (MIP) chemotherapy followed by surgical resection. All patients received postoperative mediastinal irradiation. The median survival was 26 months in the patients treated with chemotherapy plus surgery, compared with 8 months in the patients treated with surgery alone (P <.001).131 The updated 3- and 5-year survival rates for the preoperative chemotherapy arm were 20% and 17%, respectively, compared to 5% and 0%, respectively, for the surgery arm. Additionally, Rosell et al.132 observed a survival plateau in the preoperative chemotherapy group and interpreted this to imply that preoperative chemotherapy altered the natural progression of stage III disease. Depierre et al.133 reported the results of the largest randomized trial examining the role of preoperative chemotherapy in 2002. In this study, 355 patients with stage I through stage IIIA (with the exception of T1N0) were randomized to immediate surgical resection versus two cycles of mitomycin, ifosfamide, and cisplatin and two additional postoperative cycles for responding patients. In both arms, patients with pT3 or pN2 disease received thoracic radiotherapy. The median survival was 26 months in the immediate surgery arm versus 37 months with preoperative chemotherapy (p = .15, not significant [NS]). On subgroup analysis, however, a survival benefit was observed in patients with N0 or N1 disease (relative risk [RR] 0.68; p = .027), whereas there was no observed benefit to preoperative chemotherapy in the 122 patients with N2 disease (52 patients in the immediate surgery arm, 70 patients in the preoperative chemotherapy arm; RR = 1.04; p = .85). Betticher et al.134 reported the results of a multicenter phase II trial of preoperative chemotherapy in 90 patients with previously untreated, potentially operable stage IIIA (mediastinoscopically pN2) NSCLC. Patients received three cycles of docetaxel and cisplatin, with subsequent surgical resection. The pathologic complete response rate was 19% in patients undergoing tumor resection. Interestingly, 31% of patients achieved mediastinal and hilar nodal clearance (downstaged to ypN0) with this regimen. The median survival for these patients was not reached with a median follow-up of 32 months.134 In an updated report after 5 years of follow-up, the median survival still had not been reached for these patients.135 Although the data are somewhat divergent, most would recommend preoperative chemotherapy if surgical resection is planned in potentially resectable stage IIIA (pN2) disease.
Preoperative Chemoradiotherapy
Local control rates in stage III disease with chemoradiotherapy alone are inadequate. Le Chevalier et al.136 observed that the histologic 1-year local control rate was only 15% for patients with unresectable NSCLC treated to 65 Gy. A relationship has been shown between local failure and the subsequent appearance of distant metastases.137 Furthermore, improved local control in stage III NSCLC has been shown to result in a significant improvement in OS.138 The rationale for preoperative chemoradiotherapy is that surgical resection after chemoradiotherapy will optimize local control, thereby improving clinical outcomes in locally advanced disease. A phase II trial by the Southwest Oncology Group (SWOG) of induction chemoradiotherapy followed by surgical resection in 126 patients with stage IIIA/IIIB disease showed a promising 3-year survival of 26%.139 An exploratory analysis of the 27 patients with N3 disease revealed the 2-year survival rate to be 35% for the subgroup with supraclavicular nodes and 0% for the group with contralateral mediastinal nodes. Motivated by these results, an intergroup randomized phase III trial was initiated to determine the value of adding surgery to chemoradiotherapy in stage III disease with a primary end point of OS. Patients with stage T1-3 pN2 M0 NSCLC were randomly assigned to concurrent induction platin-based chemotherapy plus radiotherapy (45 Gy). If no progression, patients either underwent resection or continued radiotherapy to 61 Gy.140 A total of 202 patients were randomized to surgery and 194 to concurrent chemoradiotherapy. The median OS was 23.6 months in the trimodality arm and 22.2 months in the bimodality group (p = 0.24). For those with pN0 status at thoracotomy, the median OS was 34.4 months. PFS was better in the trimodality arm, median 12.8 months (5.3 to 42.2 months) versus 10.5 months (p = .017). An unplanned, exploratory analysis suggested that patients who underwent lobectomy in the trimodality arm had improved survival compared to matched patients receiving chemoradiotherapy; however, this result is hypothesis-generating only. One of the most important findings from this trial was the significant toxicity of right-sided pneumonectomy after induction chemoradiotherapy. Among the 29 patients who underwent a right pneumonectomy, there were 11 postoperative deaths (38%).141 Overall, this trial did not demonstrate a survival benefit for the addition of surgery to chemoradiotherapy in patients with stage IIIA NSCLC. In conclusion, the role of neoadjuvant chemoradiotherapy in stage III NSCLC remains unclear. Using a multidisciplinary approach, this strategy should be carefully tailored to the individual patient, accounting for his or her performance status, pulmonary function, extent of disease, extent of surgical resection required, and experience of the clinical team.
Adjuvant Therapy
Postoperative Radiotherapy
Locoregional recurrence after resection of NSCLC is common, occurring in approximately 20% of patients with stage I disease142,143 and in up to 50% of patients with stage III disease.135,144,145 The predominant pattern of intrathoracic failure after surgical resection is along the surgical stump or in the mediastinal nodes (Fig. 51.3). Concern over locoregional failure led to the idea that PORT in completely resected stages II and IIIA NSCLC might be beneficial because of evidence that it reduced local recurrence.146 However, the role of PORT was called into question in 1998 when the Medical Research Council published a meta-analysis of nine randomized controlled trials assessing the effect of PORT after resection.147 The PORT meta-analysis included information on 2,128 patients and 1,368 deaths. PORT was associated with a decrease in survival for patients with pN1 disease. Given the theoretical benefit of radiotherapy on local control, the detriment in survival was attributed to excessive radiotherapy-induced morbidity exceeding any benefit. There was no survival difference for pN2 patients. This analysis has been criticized for many reasons. Twenty-five percent of the patients were pN0 who did not need adjuvant therapy. There was no quality control in the radiotherapy arms, and it was felt to be inferior to modern standards; many of the patients were treated to large volumes using older Cobalt-60 equipment to fields designed under fluoroscopy. A subsequent SEER analysis provided insight to counter some of the findings from the PORT meta-analysis. In this study, over 7,400 patients with stage II/III resected NSCLC were evaluated. PORT showed an improved 5-year OS for pN2 patients (27 vs. 20%) but reduced OS for pN0 and pN1 patients.148
Additional support for the use of PORT in the modern era can be found in the Adjuvant Navelbine International Trialist Association (ANITA) trial.149 This trial randomized 840 patients at stage IB through stage IIIA between 1994 and 2000 to adjuvant chemotherapy or observation. The use of radiotherapy was not randomized; however, each center decided whether to use PORT before initiation of the study. Radiotherapy doses ranged from 45 to 60 Gy in 2 Gy fractions and were given after completion of chemotherapy. In patients with pN1 disease, PORT had an improved survival in the observation arm (median survival 25.9 vs. 50.2 months) but a detrimental effect in the chemotherapy group (median survival 93.6 months and 46.6 months). In contrast, in patients with pN2 disease, survival was improved both in the chemotherapy (median survival 23.8 vs. 47.4 months) and observation arm (median survival 12.7 vs. 22.7 months). The retrospective evaluation of the ANITA trial supports the findings from the SEER analysis that PORT may confer a benefit in pN2 NSCLC. The Lung Adjuvant Radiotherapy trial (Lung-ART) is an intergroup collaborative effort in Europe, randomizing patients with completely resected locally advanced NSCLC with mediastinal nodal involvement to observation or PORT to 54Gy. Adjuvant chemotherapy is allowed on the control arm, and pre- and/or postoperative chemotherapy is allowed on the radiotherapy arm. The trial is ongoing, and results are not yet available.
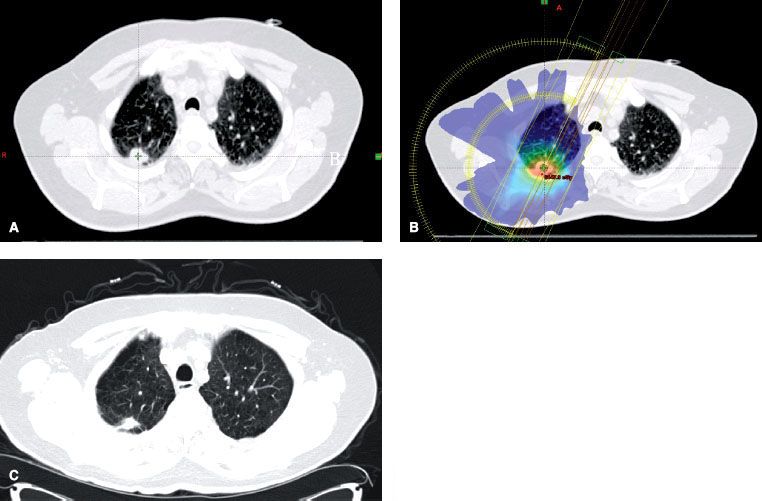
FIGURE 51.4. Stereotactic body radiation therapy (SBRT) in stage I non–small cell lung carcinoma (NSCLC). The patient was diagnosed as having T1N0M0 right upper lobe NSCLC and was treated with SBRT. A: Pretreatment tumor volume. B: Treatment plan with dose color-wash C: CT showing response 6 weeks after treatment.
Postoperative Chemotherapy
Historically, there was little evidence to support the routine use of adjuvant chemotherapy in completely resected lung cancer patients. However, benefits to chemotherapy began to emerge from clinical trials, as doubt was cast on the role for PORT. In 1995, the Non–Small Cell Lung Cancer Collaborative Group150 published a meta-analysis that showed mixed results based on the class of chemotherapeutic regimen utilized. There was decreased survival with alkylating agents, no change in survival with fluorouracil (5-FU)-based regimens, and a trend toward improved survival by 5% with cisplatin-based chemotherapy.150 This benefit was not statistically significant (p = .08); however, this publication led to numerous prospective, randomized trials investigating the role of platinum-based adjuvant chemotherapy in NSCLC.
The International Adjuvant Lung Cancer Trial (IALT) reported a statistically significant survival benefit with cisplatin-based adjuvant therapy in patients with completely resected stage I, II, or III NSCLC.151 In this trial, 1,867 patients were randomized to cisplatin-based adjuvant chemotherapy or observation. With a median follow-up duration of 56 months, patients receiving chemotherapy had a statistically significant higher survival rate (44.5% vs. 40.4% at 5 years) and disease-free survival rate (39.4% vs. 34.3% at 5 years) compared with observation. However, after 7.5 years of follow-up, there were more deaths in the chemotherapy group, and the benefit of chemotherapy decreased over time (p = .10).152
The National Cancer Institute of Canada JBR.10 trial tested effectiveness of adjuvant vinorelbine plus cisplatin versus observation in 482 patients with completely resected stage IB and II NSCLC.153 Adjuvant chemotherapy significantly prolonged OS (94 vs. 73 months) and relapse-free survival (not reached in chemo arm vs. 46.7 months) compared to observation alone. Like the IALT trial, some of the benefit diminished with longer follow-up; however, unlike IALT, the survival difference remained statistically significant. After 9 years of follow-up, adjuvant chemotherapy was found beneficial for stage II (median survival 6.8 vs. 3.6 years), although not for stage IB patients.154
In the ANITA trial, 840 patients with stage IB through stage IIIA NSCLC were randomized to adjuvant vinorelbine plus cisplatin or to observation.155 Median and 5-year OS with chemotherapy improved compared with observation. On subset analysis, this benefit was limited to node-positive patients (stage II through stage IIIA). The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis showed similar results by pooling data from five large randomized trials enrolling 4,584 patients to examine the role of cisplatin-based adjuvant chemotherapy in completely resected patients. They demonstrated a statistically significant 5.4% absolute survival benefit favoring adjuvant cisplatin.156
Postoperative Chemoradiotherapy
With the positive early results from adjuvant chemotherapy trials and prior to the publication of the PORT meta-analysis, a few groups began to explore the role of chemoradiotherapy in the postoperative setting.
One of the first multi-institutional randomized trials to investigate postoperative chemoradiotherapy was led by the ECOG. The ECOG 3590 trial randomized 488 patients with stage II through IIIA NSCLC and negative margins after surgery to either radiotherapy alone or radiotherapy plus four cycles of EP chemotherapy. Radiotherapy in both arms consisted of 50.4 Gy in 28 daily fractions. There was no difference in local recurrence or survival between the two arms.157
Before the results of ECOG 3590 were published, the RTOG embarked on a phase II combined modality study using a newer chemotherapy regimen consisting of carboplatin and paclitaxel. RTOG 9705 included 88 patients with stage II and IIIA NSCLC after surgery who received PORT with concurrent carboplatin and paclitaxel. Radiotherapy consisted of 50.4 Gy in 28 fractions with a boost of 10.8 Gy in extranodal extension or T3 lesions. The radiotherapy was administered during cycles 1 and 2. At a median follow-up of 56.7 months, median OS time was 56.3 months, with 1-, 2-, and 3-year survival rates of 86%, 70%, and 61%, respectively. The 1-, 2-, and 3- year PFS rates were 70%, 57%, and 50%, respectively. Toxicities were acceptable. When compared to previously reported studies, the RTOG concluded that these results might portend an improvement in OS and PFS with postoperative chemoradiotherapy in resected NSCLC patients.158 Promising findings were also noted in a similar study design at Fox Chase Cancer Center,132 supporting the concept that concurrent chemoradiotherapy should be formally investigated with a modern chemotherapy regimen in node-positive patients in the postoperative setting.
Summary
Adjuvant chemotherapy is accepted as standard of care for patients with node-positive (stages IIA, IIB, and IIIA) NSCLC. PORT might be beneficial in stage IIIA but is not indicated in completely resected stage I and stage II NSCLC. In practice, a patient who clinically appears to have early-stage NSCLC undergoes a gross total resection with pathology-confirmed clear margins but is unexpectedly found to have pN2 disease should receive adjuvant chemotherapy first (because of the known survival benefit) and may subsequently be considered for PORT (because of the reported local control benefit) on completion of chemotherapy.
The role of postoperative therapy for NSCLC patients at high risk for local recurrence has not been clearly established. If a patient who is clinically felt to have early-stage NSCLC undergoes surgery that results in a positive microscopic margin or residual macroscopic disease, the radiation therapy should start earlier, as local recurrence is the most common cause of failure in this group of patients.159 Chemoradiotherapy should be considered in this setting if the patient is medically fit.158,160,161
 INOPERABLE TUMORS
INOPERABLE TUMORS
Stage I/II Non–Small Cell Lung Cancer
The standard of care for a patient with operable early-stage lung cancer remains lobectomy or pneumonectomy with mediastinal lymph node dissection. However, a significant percentage of these patients cannot tolerate invasive procedures because of the comorbidities prevalent in patients with lung cancer, such as chronic obstructive pulmonary disease and poor cardiovascular health. Historically, the standard therapeutic approach for these patients has been conventionally fractionated definitive radiotherapy alone, with daily fractions delivered over a period of 6 to 8 weeks.162 More recently, a hypofractionated approach with delivery of a small number of large fractions over a short period of time has gained acceptance. This approach has most commonly been referred to as stereotactic body radiation therapy (SBRT), although recently there has been a move to rename this approach stereotactic ablative radiotherapy (SABR) to emphasize its distinct radiobiology.163
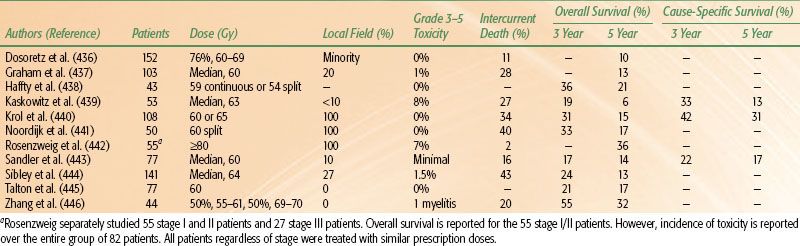
TABLE 51.6 OUTCOME BY RADIATION THERAPY DOSE AND TREATMENT VOLUME FOR PATIENTS WITH EARLY-STAGE NON–SMALL CELL LUNG CANCER