Low-Risk Prostate Cancer
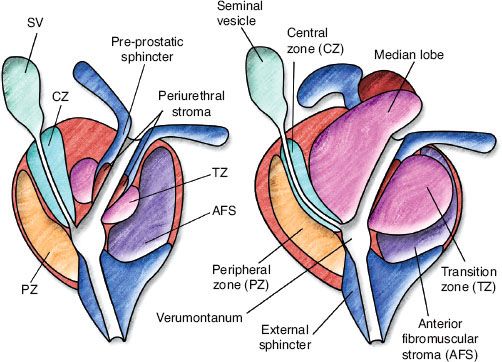
FIGURE 65.1. Zonal anatomy of the prostate. On the left, a young man with minimal transition zone (TZ) hypertrophy. Note that the preprostatic sphincter and periejaculatory duct zone (central zone of McLean) are clearly defined. On the right, an older man with TZ hypertrophy, which effaces the preprostatic sphincter and compresses the periejaculatory duct zone. AFS, anterior fibromuscular stroma; CZ, central zone; PZ, peripheral zone; SV, seminal vesicle. (From McLaughlin PW, Troyer S, Berri S, et al. Functional anatomy of the prostate: implications for treatment planning. Int J Radiat Oncol Biol Phys 2005;63:479–491; with permission from Elsevier.)
 ANATOMY
ANATOMY
Gross Anatomy and External Architecture
The prostate gland is an ovoid-shaped structure composed of fibrous, glandular, and muscular elements. It is located in the pelvis, adjacent to the rectum, bladder, dorsal, and periprostatic venous complexes, pelvic sidewall musculature, the pelvic plexus, and cavernous nerves. Because of its shape, the prostate and the rectum curve away from each other as two convex surfaces. The prostate surrounds segments of the urethra before it passes through the genitourinary diaphragm (GUD; Fig. 65.1). The male urethra is composed of five segments: the pre-prostatic urethra adjacent to the pre-prostatic sphincter; the prostatic urethra, which is from the verumontanum to the GUD; the membranous urethra as it courses through the GUD, which is surrounded by the external sphincter; the bulbar urethra in the penile bulb; and the penile urethra as it passes through the corpus spongiosum. The paired seminal vesicles are situated posterosuperiorly to the prostate gland and secrete seminal fluid into the bilateral ductus deferens as they become the ejaculatory ducts. These ducts transverse the prostate to join the urethra at the verumontanum (Fig. 65.1, right). At this point, the urethra changes its angulation by bending 30 to 40 degrees anteriorly.
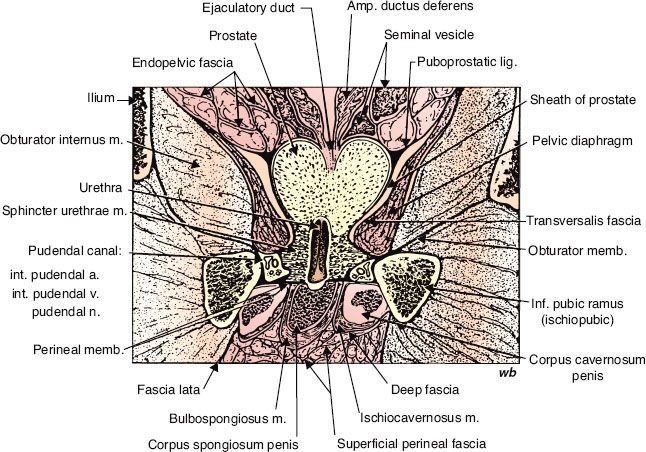
The prostate is contained within a thin, fibrous adherent capsule that is structurally continuous with the stroma of the gland. The apex of the gland rests above the GUD. The GUD surrounds the membranous sphincter and may vary in length and thickness. The puboprostatic ligaments extend anteriorly from the surface of the gland to the pubic symphysis. The prostate is separated from the rectum posteriorly by Denonvilliers’ fascia (retrovesical septum), which attaches above to the peritoneum and below to the GUD. It is this portion of the prostatic fascia that restricts posterior extension of prostatic carcinoma into the rectum. The lateral margins of the prostate are usually delineated against the levator ani muscles, forming the lateral prostatic sulci.
The anterior aspect of the prostate and the lateral pelvic floor are covered with the periprostatic fascia (Fig. 65.2). The endopelvic fascia lateral to the prostate gland contains neurovascular structures, including the venous plexus of Santorini, which is the primary drainage for the penis. This venous network, also referred to as the dorsal vein complex, covers the anterolateral surfaces of the prostate. The primary arterial blood supply to the prostate is via branches of the internal pudendal, inferior vesical, and middle hemorrhoidal arteries. The internal pudendal arteries also provide the blood flow to the penis. The nerves originate from the pelvic plexus, containing both sympathetic and parasympathetic fibers, and are distributed to the prostate, seminal vesicles, and the corpora cavernosa of the penis and urethra.
The prostatic apex is definable on diagnostic imaging and is important anatomically for radiation therapy treatment planning. Although the apex and the GUD, which surrounds the membranous sphincter, are easily visualized on coronal magnetic resonance imaging (MRI), it is important to recognize that the level of the GUD from the apex to the penile bulb may vary because the absence of an apical capsule contributes to the difficulty in discrimination of the gland from the GUD during computed tomography (CT)-based treatment planning. Delineation of the neurovascular bundle is also limited on CT imaging.
FIGURE 65.2. Frontal section of male pelvis at right angles to perineal membrane. (From Oelrich TM. The urethral sphincter muscle in the male. Am J Anat 1980;158:229–246;reproduced with permission of John Wiley & Sons, Inc.)
Prostatic Zonal Anatomy
Zonal anatomy has essentially replaced lobar anatomy of the prostate. There are four zones of the prostate (Fig. 65.1): the peripheral zone (PZ), transition zone (TZ), central zone, and anterior fibromuscular stroma zone. The central zone that surrounds the ejaculatory ducts has marked histologic differences from the PZ. It is the PZ, extending across the entire posterior surface of the gland that is palpated on rectal examination and is the location of most prostate cancers. The TZ is the location of benign prostatic hypertrophy. The anterior fibromuscular zone consists of an anterior band of fibromuscular tissue contiguous with bladder muscle and external sphincter. In young men, the PZ is the prominent zone, whereas the TZ becomes the dominant zone with age. It is important to note that there is no “median lobe” zone in this nomenclature, although such a “lobe” may be present in some prostate cancer patients and may have important implications for treatment planning and treatment selection. Histologically, the median lobe arises from the TZ or periurethral stroma, with varying proportions of fibrous, glandular, and muscle tissue.
Prostate Physiology
Histologically, the prostate consists of compound tubuloalveolar glands lined by two layers of cells. The glands are embedded in connective tissue comprising collagen and abundant smooth muscle that constitutes the prostatic stroma. This fibromuscular stroma functions both to control micturition by acting as a sphincter of the urethra and to express acidic prostatic secretions into the urethra by contracting during ejaculation.
The major function of the prostate is the production of seminal fluid that protects and nourishes the sperm after ejaculation. The prostate contributes approximately 30% to the seminal fluid, and the seminal vesicles, testicles, and bulbourethral glands provide the remaining 70%. Enzymes, including acid phosphatase and prostate-specific antigen (PSA), are secreted into the seminal fluid. PSA is a serine protease that is involved in the liquefaction of the seminal coagulum. Because PSA is produced primarily by benign and malignant prostatic epithelial cells and normally found at low concentrations in the serum, it is useful for prostate cancer screening and posttreatment monitoring of disease status.
The synthetic activity and growth of the prostate gland is regulated by androgens. The primary circulating androgen is testosterone. In the prostatic stroma, testosterone is converted to its active and more potent form, α-dihydrotestosterone, by 5-α-reductase. Secretory epithelial cells and stromal cells have intracellular androgen receptors. Dihydrotestosterone forms a complex with the dihydrotestosterone-binding domain of the androgen receptor, altering the structure of the DNA-binding domain such that it can reversibly bind DNA sequences known as androgen response elements in promoter or enhancer regions of androgen-regulated genes. The response includes stimulation of cell division, inhibition of apoptosis (programmed cell death), or cellular differentiation. In secretory epithelial cells, testosterone stimulation may result in the production and secretion of prostatic fluid. These mechanisms are tightly regulated at many levels, from the hypothalamus secreting luteinizing hormone–releasing hormone to maintain testosterone levels in the blood, to the local regulation of 5-α-reductase in the prostate stroma. All of these factors acting at the same time determine the balance between cellular proliferation, cell death, and differentiation of a prostatic epithelial cell.
TABLE 65.1 KNOWN OR SUSPECTED RISK FACTORS FOR PROSTATE CANCER
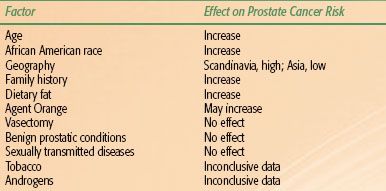
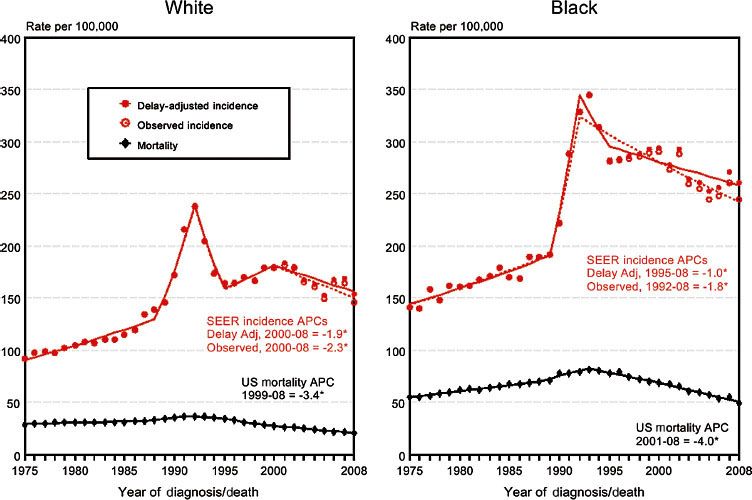
FIGURE 65.3. Surveillance, Epidemiology, and End Results (SEER) prostate cancer incidence and mortality rates by race, United States, 1975–2008. (SEER Cancer Statistics Review, 1975–2008. Available at: http://seer.cancer.gov.)
 EPIDEMIOLOGY AND RISK FACTORS
EPIDEMIOLOGY AND RISK FACTORS
Clinical Incidence
Adenocarcinoma of the prostate is the most frequently diagnosed visceral cancer of men in the United States, accounting for 33% of non-skin cancers. The lifetime risk for American White and African American men is 18% and 21%, respectively. This corresponds to a respective lifetime risk of prostate-specific mortality of 3% and 5%.1,2 After large annual increases from 1988 to 1992, coinciding with the introduction of the PSA screening test, prostate cancer incidence in the United States leveled off, then showed modest decreases of approximately 1.9% per year between 2000 and 2008 (Fig. 65.3). Incidence rates between 2004 and 2008 were approximately 153 per 100,000 men.2
Perhaps because of widespread use of PSA screening and effective early treatment for localized disease, the age-adjusted death rates have begun to decrease. It was estimated that 241,740 new cases of prostate cancer would be diagnosed in 2012, but only approximately 28,170 patients would die of the disease.2 This compares with 189,000 new cases and 30,200 estimated deaths in 2002.1 Despite these encouraging trends, in 2008 carcinoma of the prostate remained the second greatest cause of male cancer mortality behind cancer of the lung and bronchus.3
Of the known or suspected risk factors for prostate cancer, the most important is age (Table 65.1).4 The median age at diagnosis is 68 years, and the disease incidence escalates sharply with increasing age. The incidence among men age 40 to 59 years is 1 in 38, increasing to 1 in 15 among men age 60 to 69 years and 1 in 8 among those age 70 years and older. According to autopsy data, 70% of men older than 80 years of age and 40% of men older than 50 years of age have pathologic evidence of cancer in the prostate.5
Although the risk for development of histologic evidence of cancer in the prostate is fairly constant across countries and races, there is considerable variability in the incidence of clinically evident disease and mortality among different populations worldwide and in the United States.6
The highest rates of prostate cancer are in Scandinavia, where it is the leading cause of male cancer death. The lowest recorded rates are in Asia. In the United States, incidence and mortality are higher among African Americans. A 30- to 50-fold difference in risk between African American men at the highest end of the spectrum and native Japanese at its lowest end has been reported. The mortality rates of prostate cancer in Japan dramatically increased from 1960 to 2000 in all age groups before showing modest but sustained decreases from 2000 onward.7
Risk Factors
Hormonal Influences
It is relatively well established that androgenic influences over time affect prostate carcinogenesis and disease progression.8 Generally, androgens are required for the development of prostate cancer, and it has been noted that men deficient in 2,5α–reductase are rarely diagnosed with benign prostatic hypertrophy or prostate cancer. In a study of 1,008 men, there was a positive correlation with plasma androstenedione levels and the development of prostate cancer.9 However, Gann et al.10 found no clear association between individual hormone levels, including dihydrotestosterone, and the incidence of prostatic cancer. Yet, these investigators noted that high levels of testosterone in combination with low levels of the serum protein that binds testosterone, sex hormone–binding globulin, correlated with a higher risk of prostate cancer. Meikle et al.11 observed a higher sex hormone–binding globulin level and a higher rate of testosterone synthesis in men with prostate cancer compared with control subjects.
The most conclusive evidence supporting the hormonal influence in the development of prostate cancer comes from two large, randomized trials evaluating the use of 5α-reductase inhibitors in chemoprevention. The Prostate Cancer Prevention Trial was the first large-scale, population-based trial testing the hypothesis that treatment with finasteride, which lowers intraprostatic dihydrotestosterone levels, prevents prostate cancer.12 In this trial, a total of 18,882 men ≥55 years old and with normal digital rectal examination (DRE) and PSA level ≤3.0 ng/mL were randomized to 7 years of finasteride (5 mg/day) or placebo. This study found that the prevalence of prostate cancer was reduced by 25% in men taking finasteride compared to placebo, with the prevalence of Gleason sum 7 to 10 tumors higher in the former group. The Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial randomized 8,231 men at high risk for prostate cancer (age 50 to 60 years with a PSA of 2.5 to 10.0 or age ≥60 years with a PSA of 2.5 to 10.0 and a negative prostate biopsy within 6 months of enrollment) to dutasteride (0.5 mg daily) or placebo.13 At 4 years, a relative risk reduction in the diagnosis or a subsequent prostate cancer of 22.8% was noted in men taking dutasteride. However, a trend toward increased Gleason 8 to 10 tumors was noted among men taking dutasteride. For both trials, it remains controversial whether this increased risk of high-Gleason tumors is real or artifactual. However, the U.S. Food and Drug Administration has declined to add chemoprevention of prostate cancer as an indication for finasteride and dutasteride.14
Dietary Influences
The development of prostate cancer may be attributed to both familial and environmental factors. Epidemiologic studies strongly suggest that a diet deficient in certain micronutrients is an important environmental risk factor. This has been supported through migration studies that indicate higher rates of prostate cancer in Asian men living in the United States compared with their counterparts in Japan or China.15,16 It has been postulated that nutritional factors play a role in stimulating the progression of microscopic disease. Salient features of the “Western diet” that differ from the traditional Asian diet are high fat intake and low soy consumption. A Western diet has been associated with increased production of both androgens and estrogens and a vegetarian diet with lower levels.17 Numerous case–control and cohort studies demonstrated an association between increased dietary fat intake and a higher risk of prostate cancer.18 Diets high in fat content may increase the relative risk of prostate cancer by a factor of 1.6 to 1.9.19,20 In addition, several studies showed that men with diets high in fiber and presumably lower in fat have a decreased risk of prostate cancer.21,22
One prospective study found that the type of fat intake was directly related to risk of prostate cancer.23 Red meat represented the food group with the strongest positive association with advanced cancer, with a relative risk of 2.64. Fat from dairy products (with the exception of butter) or fish was unrelated to risk. When analyzed by fatty acid type, only α-linolenic acid (an omega-3 fatty acid found in red meats and butter), and not linoleic acid (omega-6 fatty acid found in fish oil), was implicated, with an increase in risk for prostate cancer of more than threefold. Conversely, a subsequent Canadian case-controlled study suggested that saturated fat consumption, not α-linolenic acid consumption, may play a role in prostate cancer progression.24 Finally, a Swedish study of 406 men with prostate cancer and 1,208 without it (control subjects) demonstrated that body mass index and total amount of food consumed were independent risk factors.25
Plant-based foods and their products, such as soy, tomatoes, cruciferous vegetables, and certain nutrients, are favorably associated with prostate cancer. It is believed that these dietary factors contribute to antioxidant effects against DNA and cell damage.
Several vitamins and trace nutrients, including selenium, vitamin E, and vitamin C, have garnered attention as potential protective agents. Selenium is an essential trace nutrient that humans obtain through their diet of plants (related to soil composition; highest in Brazil nuts) and animal products (highest in seafood) and is present in nutritional supplements. Early experimental and epidemiologic data supported the anticarcinogenic effects of selenium through apoptotic, angiogenic, or antioxidative pathways.26 The Nutrition Prevention Trial identified a 50% reduction in prostate cancer risk in men blindly and randomly assigned selenium supplements compared with placebo.27 Promising data for vitamin E as a protective agent was reported in the Finnish Alpha-Tocopherol, Beta-Carotene Cancer (ATBC) Prevention Study, which evaluated supplementation with vitamin E and beta-carotene in male smokers for chemoprevention of lung cancer.28 Secondary analysis suggested a 32% reduction in prostate cancer risk among men randomized to receive vitamin E. However, a large, prospective, randomized trial failed to confirm these findings for selenium or vitamin E. The Selenium and Vitamin E Cancer Prevention (SELECT) Trial randomized 35,533 men to four arms: selenium (200 μg/day), vitamin E (400 IU/day rac-α-tocopheryl acetate), both selenium and vitamin E, or placebo only.29 With a minimum follow-up of 7 years, the study identified no reduction in risk of prostate cancer with either vitamin E or selenium supplementation but identified an increased hazard ratio for development of prostate cancer among men randomized to vitamin E alone of 1.17. Vitamin E was contemporaneously evaluated in the randomized Physicians’ Health Study II, which failed to demonstrate a reduction in prostate cancer incidence by supplementation with vitamin E or C.30
The beneficial effects of soy are attributed to isoflavones, one of several plant pigments found in soybeans. Isoflavones are a type of phytoestrogen—compounds that have weak estrogenic, antiestrogenic, and antioxidant effects that may all be protective against progression of prostate cancer in humans. These isoflavones, most significantly genistein and daidzein, have been shown to inhibit the growth of prostate cancer cell lines in nude mice.18 In particular, genistein has been shown to be a potent inhibitor of several steroid-metabolizing enzymes, such as aromatase, 5α-reductase, and 17β-hydroxysteroid dehydrogenase, as well as enzymes that are crucial to cellular proliferation, such as tyrosine kinase and topoisomerases I and II. Genistein is also an inhibitor of angiogenesis. It is estimated that Japanese men consume approximately 20 mg of isoflavones per day, whereas for Western men the daily consumption is <1 mg/day. This is reflected in a mean plasma concentration of genistein of 180 ng/mL in Japanese men, compared with a level of <10 ng/mL for Western men.31
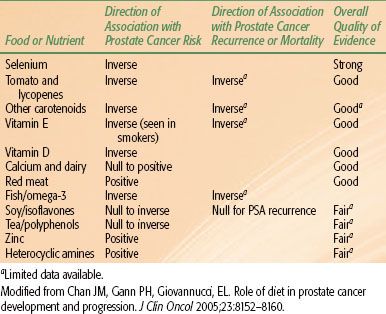
Another protective nutrient is lycopene, prevalent in the Western diet, a carotenoid that is present in tomatoes, processed tomato products, and other fruits. It is one of the most potent antioxidants among dietary carotenoids. Although the antioxidant properties of lycopene are believed to be primarily responsible for its beneficial effects, other mechanisms may also be involved. In a review of 72 epidemiologic studies that investigated a link between cancer risk and consumption of tomato products, 57 linked tomato intake with a reduced risk; in 35 of those studies, the association was considered statistically significant.32 Two large, prospective studies reported a decrease in prostate cancer risk with higher tomato product consumption.33,34 Tomatoes were one of only four specific food items associated with significantly reduced prostate cancer risk in a prospective study of 14,000 Seventh-Day Adventist men.34 A prospective study examined the relationship between the plasma concentration of several antioxidants and the risk for prostate cancer, using plasma samples obtained in the Physician’s Health Study. Higher serum and tissue lycopene levels were found to be inversely related to the incidence of prostate cancer development.35 In a recent meta-analysis of 21 studies, high intake of tomatoes was associated with a 10% to 20% risk reduction in prostate cancer, with lower risk due to cooked versus raw tomatoes.36 A summary of various epidemiologic studies indicating associations of various dietary factors to prostate cancer development and morality is shown in Table 65.2.
Familial Associations
A large cohort study of the Utah Mormon population demonstrated a positive family history of prostate cancer in 6.6% of families of probands with prostate cancer and only 2.2% of families of probands without prostate cancer.37 A subsequent study using data from the Utah State Cancer Registry reported a familial relative risk of prostate cancer of 2.2.38 Multiple studies have confirmed these findings, including a large case–control study from Johns Hopkins.39 Extensive cancer pedigrees were obtained from 691 prostate cancer cases and 640 spouse control subjects showing a twofold increased risk in men with a family history of prostate cancer in a single first-degree relative. There was a fivefold risk if there were two affected relatives, and the relative risk rose to 11 for three first-degree relatives with prostate cancer.
In a Canadian study, the frequency of prostate cancer detected in men who had a first-degree relative with a history of prostate cancer was 2.6 times greater than for men without such a history.40 Aprikian et al.,41 in a study of 2,968 patients, noted that prostate cancer was detected in 40% (1,300 patients) of men with a family history of prostate cancer, compared with 29% of 769 men without a family history (p < .0001). In a review of the epidemiology of prostate cancer, Giovannucci33 reported that approximately 9% of cases may be attributed directly to a family history, although this may be as high as 43% among men younger than 55 years of age.
Despite the familial clustering of prostate cancer observed in these epidemiologic studies, causation cannot be inferred, given the shared environmental factors among family members. Segregation analyses of cancer in multiple family members have been used to examine the role of genetic factors and inheritance patterns in prostate cancer. Segregation analysis is a statistical method used to determine the best-fitting model of inheritance for a particular disease in a study population. The largest segregation analysis of prostate cancer families suggested that the familial pattern was best explained by Mendelian inheritance of a rare, autosomal dominant gene in a subset of men with early-onset prostate cancer.42 The allele is highly penetrant, accounting for cancer by age 85 years in 88% of carriers compared with only 5% of noncarriers. Although this gene appears to be responsible for many of the early-onset cases, only 9% of all prostate cancer cases are in patients with this genetic predisposition, a percentage similar to that seen in both hereditary breast and colon cancers. Twin studies have also been used in the analysis of prostate cancer inheritance and have shown four to five times higher concordance rates for monozygotic twins.43
TABLE 65.2 NUTRITIONAL RISK FACTORS FOR PROSTATE CANCER INCIDENCE, RECURRENCE, AND MORTALITY
Genetic and Molecular Influences
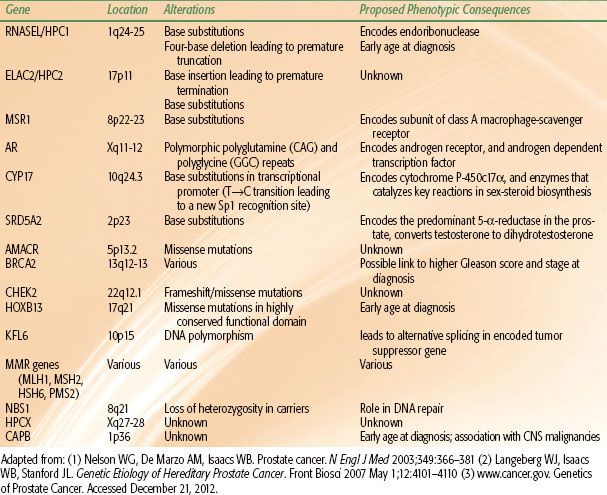
Researchers are now focusing on the molecular level to identify genetic alterations that may be involved in the multistep process of carcinogenesis. Progress in cytogenetic studies using polymerase chain reaction (PCR)–based polymorphism analysis has facilitated the identification of regions of the genome associated with various types of cancer. Linkage analysis is used to determine whether there is an association between a particular genetic defect and clinical disease. There is ongoing investigation into the role of chromosomal deletions, oncogenes, and tumor suppressor genes in the initiation and progression of prostate cancer. Emerging data from analysis of DNA from high-risk families suggest that specific high-risk alleles exist for prostate cancer, as they do for other tumors. A major susceptibility locus for prostate cancer on the long arm of chromosome 1 (1q24-25) was identified through a genome-wide scan.44 The gene, HPC1 (hereditary prostate cancer 1), has been linked to families with multiple members affected with an early average age at diagnosis.45 However, this association has not been identified in all studies. Subsequent studies have sought to identify additional susceptibility loci and germline mutations implicated in familial prostate cancer. A recent study by Ewing et al.46 identified a recurrent mutation in HOXB13, a homeobox transcription factor gene involved in prostate development on the long arm of chromosome 17 (17q21-22) implicated in early-onset, familial prostate cancer. A number of other candidate susceptibility genes have been studied,47 several of which are summarized in Table 65.3.
Research delving into the molecular physiology of the prostate gland has also uncovered specific DNA sequences that may be related to the occurrence and progression of prostate cancer. It is well known that prostate cancer cells, like their normal counterparts, are usually sensitive to androgens, and their growth depends on androgen-stimulated cell division. Prostate cell growth is controlled by the interaction of circulating androgens, such as testosterone and dihydrotestosterone, with the androgen receptor. The androgen receptor gene contains a polymorphic CAG repeat sequence that encodes the portion of the receptor involved in DNA transcription. The length of the CAG repeat sequence was found to be inversely proportional to the activity of the androgen receptor; therefore, shorter CAG repeat sequences may be related to prostate cancer growth.48 Giovannucci et al.,49 in an analysis of the activity of the androgen receptor in men with prostate cancer, found that a shorter CAG repeat sequence in the androgen receptor gene predicts higher grade and more advanced stage of prostate cancer at diagnosis, as well as metastasis and mortality from the disease. Other studies found that the prevalence of short CAG repeats is higher among African Americans than Whites50 and that these sequences are longest among Chinese men.8 These findings may partly explain the higher risk for development of prostate cancer among African Americans and the lower risk in Asian countries.
In addition to these genetic alterations identified in prostate cancer, epigenetic changes such as abnormal DNA methylation have also been observed and may play a role in upregulation or loss of expression of genes.51 Millar et al.52 observed that abnormal methylation of specific sites throughout the genome of prostate cancer cells leads to loss of glutathione S-transferase P1 (GSTP1) gene expression. The GSTP1 gene product is an enzyme that provides protection to mammalian cells against electrophilic metabolites of carcinogens and reactive oxygen species. Loss of GSTP1 may lead to a transition between proliferative inflammatory atrophy and prostatic intraepithelial neoplasia or prostate cancer.53
TABLE 65.3 PARTIAL LIST OF PROSTATE CANCER SUSCEPTIBILITY GENES AND CANDIDATE GENES
Other Risk Factors
Chronic or recurrent inflammation may have a role in the development of prostate cancer, as has been recognized in many other human cancers. Although various microbial organisms have been identified to infect prostate tissue, the specific offending pathogen causing prostatitis has not been isolated. However, the specific cause may not be necessary, as it is the host inflammatory response to an infection rather than the infectious agent itself that may lead to prostate cancer. In one large population-based study, prostate cancer risk was increased in men with a history of gonorrhea or syphilis (odds ratio, 1.6; 95% confidence interval, 1.2 to 2.1).54 A criticism of such epidemiologic studies is the bias that men with symptomatic prostatitis compared with men without it are more likely to seek out care with urologists, have an increased serum PSA test, and have prostate biopsies.53
Several commonly used medications have gained attention as potential chemopreventive agents, with particular interest in 5-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins). Data for the effects of statin use on subsequent risk of prostate cancer are conflicting, with some observational cohort studies and retrospective analyses suggesting a reduced risk of advanced prostate cancer,55 reduced risk of death from prostate cancer,56 and lower rates of relapse following radiotherapy57 or radical prostatectomy (RP).58 However, several large meta-analyses failed to identify a relationship between statin use and prostate cancer risk,59,60 and no prospective, randomized studies have confirmed these observations.
Other risk factors for prostate cancer have been implicated but not corroborated. Several reports suggested an association between vasectomy and prostate cancer. In a prospective study of 10,055 vasectomized men and 37,800 nonvasectomized men, Giovannucci et al.61 found an increased risk of 1.85 in the vasectomized men. In a retrospective study of 14,607 vasectomized or nonvasectomized men, the increased risk was 1.56.62 A recent population-based case–control study of 923 new cases of prostate cancer among men aged 40 to 74 years from the New Zealand Cancer Registry showed no association between prostate cancer and vasectomy.63 Although there appears to be no definite etiologic relationship, it is possible that men undergoing vasectomy are more conscious of health care and more likely to be screened for prostate cancer.64 Circumcision has not been correlated with development of prostate cancer.65
Armenian et al.,66 in a study of 296 patients with benign prostatic hyperplasia diagnosed either histologically or clinically and 299 age-matched control subjects observed from 7 to 27 years, found the incidence of prostatic cancer to be 3.7 times higher in the hyperplasia group than in the control group. This association was not observed by others.67
Some studies correlated smoking with increased risk of prostate cancer,68 whereas another study did not find a significant correlation.69 In one analysis of 359 patients,70 a greater tumor-specific mortality rate among smokers than nonsmokers with stage A and D tumors was observed. No occupational factors have been confirmed as risks, but some evidence suggests that occupational exposure to cadmium and some aspects of farming may increase risks moderately, although these factors would account for only a small proportion of the total cases. Japanese men exposed to atomic bomb explosions in Hiroshima and Nagasaki have not had a significantly higher incidence of prostatic cancer.71
 NATURAL HISTORY
NATURAL HISTORY
Local Growth Patterns
The studies of prostate morphology conducted by McNeal72 showed that almost all prostatic carcinomas (>70%) develop in the PZ of the prostate, whereas benign prostatic hyperplasia (>90%) arises from the TZ. In addition, examination of prostatectomy specimens revealed that small tumors tend to occur in the anteromedial gland, adjacent to the fibromuscular stroma, whereas larger, more advanced T-stage tumors are often located in the posterior gland near the prostatic capsule.73,74
Multifocality is characteristic of prostate cancer. On DRE, there may be one nodule or many, located unilaterally or bilaterally. Histologic and molecular studies of prostatectomy specimens of patients with prostate cancer have revealed that most contain a dominant or index tumor and one or more spatially separate, often heterogeneous tumors.75–78 Jewett79 reported that multiple foci of disease were found throughout the prostate in 77% of prostatectomy specimens. Wise et al.80 noted that only 17% of 486 patients treated by radical retropubic prostatectomy had one carcinoma detected by 3-mm step-section histologic examination. Of the 83% with multifocal disease, secondary cancers were mostly small; 58% were <0.5 cm3 in volume. Qian et al.,81 using fluorescence in situ hybridization to detect chromosomal abnormalities, found that an increasing incidence of chromosomal anomalies among specimens was positively correlated with progression from high-grade prostatic intraepithelial neoplasia to prostatic carcinoma. When lymph node involvement was present, there was usually evidence of one or more foci of the primary tumor sharing chromosomal anomalies with associated lymph node metastases, suggesting that just a single focus of carcinoma may give rise to metastases. This heterogeneity and multicentricity may account for the discrepancy between needle-biopsy Gleason score and the grade determined from the dominant tumor in the prostatectomy specimen.
Tumors arising from the TZ tend to demonstrate a lower frequency of extracapsular extension and may harbor large volumes of disease with relatively high PSA levels but remain confined to the prostate. Despite a high PSA value (≥10 ng/mL), these tumors should be considered to have a favorable prognosis and be managed accordingly. Noguchi et al.82 described the histologic characteristics of 148 cases of TZ prostate cancer from RP specimens. Seventy percent were clinical stage T1c, with a preoperative serum PSA of 10 ng/mL or greater in almost two-thirds of cases. Only 63% had a positive initial prostatic biopsy. On pathologic review, 80% had organ-confined disease, and more than one-third of cases had a cancer volume >6 mL. When compared with 79 PZ cancers matched by volume, there were no differences in percentage Gleason grade 4/5, serum PSA, or prostate weight, although differences in clinical stage T1c to T2c and organ-confined cancer were highly significant. The actuarial 5-year PSA relapse–free survival rate was 71.5% among men with TZ cancer, compared with 49.2% for those with PZ cancer.
PZ cancers tend to spread along the capsular surface of the gland and may extend through the capsule of the gland, invade seminal vesicles and periprostatic tissues, and involve the bladder neck or the rectum. Clinical stage closely correlated with risk of extracapsular extension and disease progression.83–85 The incidence of microscopic tumor extension beyond the capsule of the gland (at the time of RP) in patients with clinically organ-confined disease ranges from 8% to 57%.86,87 Oesterling et al.,88 in an analysis of patients with stage T1c disease treated with RP, noted that 53% had pathologically organ-confined tumors, 35% had extracapsular extension, and 9% had seminal vesicle invasion. Of the last group, 66% had positive surgical margins, an incidence comparable with that for clinical stage T2 tumors. In a similar group of patients with T1c tumors, Epstein et al.89 found that 34% had established extracapsular extension, 6% had seminal vesicle invasion, and 17% had positive surgical margins.
Pretreatment serum PSA is also predictive of extraprostatic extension and seminal vesicle invasion. The rate of organ-confined prostate cancer ranges from 53% to 67% for men with a PSA level between 4 and 10 ng/mL and from 31% to 56% for men with a PSA level between 10 and 20 ng/mL.90–92 D’Amico et al.,93 in a pathologic evaluation of 347 RP specimens, reported that none of 38 patients with PSA of ≤4 ng/mL had seminal vesicle involvement, in contrast to 6% of 144 patients with PSA of 4 to 10 ng/mL, 11% of 101 with PSA of 10 to 20 ng/mL, 36% of 45 with PSA of 20 to 40 ng/mL, and 42% of 19 with PSA of >40 ng/mL. The incidence of positive surgical margins for these PSA subgroups was 11%, 20%, 33%, 56%, and 63%, respectively. The incidence of seminal vesicle involvement also is associated with the level of PSA, the Gleason score, and the clinical stage.94,95 Seminal vesicle involvement has been observed in from 10% of patients with A2 tumors to 30% of the patients with B2 lesions.96,97
Roach98 proposed formulas based on analysis of RP specimens to estimate the probability of extracapsular extension (ECE+) and seminal vesicle involvement (SV+):
ECE+ = 3/2 PSA + (Gleason score − 3) é 10
SV+ = PSA + (Gleason score − 6) é 10
Regional Lymph Node Involvement
Tumor size and degree of differentiation affect the tendency of prostatic carcinoma to metastasize to regional lymphatics.99,100 With an increasing number of patients being diagnosed in earlier stages (as a result of screening PSA), there has been a decreased incidence of lymph node metastases in patients with clinical stage T1c and T2 tumors.101 In the low-risk prostate cancer patients, the risk of lymph node involvement is generally considered <10%.
Several groups90,102–107 have developed models based on clinical or pathologic data that predict the risk of lymph node metastases. This information is important to decide whether a prostate cancer patient should be subjected to a staging lymphadenectomy (including laparoscopic technique) or considered for irradiation of the pelvic lymph nodes. Partin et al.108 analyzed data from 703 patients and generated a nomogram for predicting nodal metastases based on three factors: clinical stage, preoperative PSA, and tumor biopsy grade. This model was validated in a larger multicenter study of 7,014 men and accurately predicted nodal metastases in 78% of patients.109 The negative predictive value was 99%. Bluestein et al.104 tested a model based on multivariate logistic regression analysis on 1,632 patients who underwent pelvic lymphadenectomy at the Mayo Clinic for staging of prostate cancer. Using this method, they determined that 29% of the patients with clinical stage T1a to T2c disease would have been spared pelvic lymphadenectomy with only a 3% rate of missed nodal metastases. Bishoff et al.103 reported similar results demonstrating that 20% to 63% of patients with prostate cancer could be spared pelvic lymphadenectomy when accepting a 2% to 10% risk for missed nodal metastasis. These results suggest that many patients can be spared pelvic lymphadenectomy solely by analyzing preoperative PSA, Gleason grade, and clinical stage, without incurring an unacceptable risk for failing to identify regional metastasis.110
Stock et al.,95 in a study of 99 patients who underwent laparoscopic lymph node dissection, correlated incidence of positive nodes with PSA, Gleason score, stage, and involvement of seminal vesicles. None of the patients with a Gleason score of 4 or lower, even those with PSA of >20 ng/mL, had positive pelvic lymph nodes, and 8% in the group with Gleason scores of 5 or 6 and PSA levels of 4 to 10 ng/mL had positive nodes. However, the incidence of positive lymph nodes increased significantly (to 24%) in patients with PSA of >20 ng/mL. These results are similar to those reported in patients treated with RP.104,108
In an analysis of 2,144 patients treated at two institutions, Rees et al.111 noted that only 30 (2.2%) of 1,390 patients with a negative DRE and either PSA of 5 ng/mL or less, Gleason score of 5 or less, or a combination of PSA of <25 ng/mL and Gleason score of ≤7 had pelvic lymph node metastases.
Roach98 suggested a formula based on pathologic findings in prostatectomy specimens to estimate the incidence of metastatic pelvic lymph nodes (Nodes+):
Nodes+ = 2/3 PSA + (Gleason score − 6) é 10
Prognosis is closely related to the presence of regional lymph node metastases; patients with positive pelvic lymph nodes have a significantly greater probability (>85% at 10 years) for development of distant metastasis than those with negative nodes (<20%).112 However, a single nodal metastasis is not an unfavorable prognostic sign. In a study by Cheng et al.,113 322 patients with positive lymph nodes after RP and bilateral pelvic lymphadenectomy were followed for a median of 6 years. Patients with prostate carcinoma who had multiple regional lymph node metastases had increased risk of death from disease, whereas patients with a single positive lymph node appeared to have a more favorable prognosis after RP and immediate adjuvant hormonal therapy. Prout et al.,114 in 92 patients with various stages of prostatic carcinoma, noted solitary lymph node metastasis in 11 (34%) of 32 patients with positive nodes. Bilateral pelvic lymph node involvement was present in 14 (58%) of 24 patients who had more than one metastatic lymph node. Only 2 (18%) of 11 patients with a single metastasis showed tumor progression, compared with 15 (76%) of 21 with multiple lymph node involvement. Golimbu et al.115 noted a 10-year survival rate of 50% in patients with a single positive lymph node, compared with 20% for those with multiple lymph node involvement.
The prognostic significance of multiple involved lymph nodes should be considered with the extent of lymphadenectomy. Several studies have evaluated the anatomic extent of pelvic lymph node dissection on outcome.116,117 At Johns Hopkins University Hospital, two surgeons performed 4,000 RP with or without an extended lymph node dissection. The extended dissection removed more lymph nodes (mean 12 vs. 9; p < .0001) and detected more lymph node–positive prostate cancer (3.2% vs. 1.1%; p < .0001) than more limited procedure. If disease was found involving <15% of the extracted lymph nodes, extended lymph node dissection resulted in a more favorable 5-year PSA progression-free survival. Thus, in certain subgroups, an extended dissection may be beneficial. In another study, the number and percentage of involved lymph nodes correlated with recurrence-free and overall survival.117 These results need additional validation in prospectively controlled studies.
 CLINICAL PRESENTATION AND DIAGNOSTIC WORKUP
CLINICAL PRESENTATION AND DIAGNOSTIC WORKUP
Screening Methods and Markers
Although DRE is still an essential element in screening and assigning clinical stage, only 25% to 50% of men with an abnormal DRE have cancer on biopsy. Moreover, because carcinoma of the prostate can be asymptomatic until attaining a significant size, if a patient presents with a palpable tumor there is a significant risk that there already may be locally advanced or metastatic disease. With the advent of PSA screening, the diagnosis of prostate cancer may precede palpable disease on DRE and the symptoms of urinary obstruction or metastatic disease by many years. DRE is associated with 70% sensitivity and 50% specificity.118 In fact, 70% of cancers detected by PSA screening are confined to the prostate, and 40% of cancers detected by PSA are not palpable.87
PSA, initially identified and purified from prostatic tissue by Wang et al.119 in 1979, is a protein with a molecular weight of 33,000. PSA is detected not only in prostatic tissue (normal tissue, benign hyperplasia, and malignant tumors) and in seminal fluid, but also in the sera of patients with prostatic cancer. It is localized in the cytoplasm of ductal epithelial cells and in secretory materials in ductal lamina.120 PSA has been detected with immunohistochemical techniques in pancreas and salivary glands and in women; therefore, it is not absolutely specific for prostatic epithelium.121
Widespread use of PSA screening has come into question following publication of two landmark screening trials. The European Randomized Study of Screening for Prostate Cancer (ERSPC) randomized 162,387 men age 50 to 74 years to PSA screening an average of once every 4 years or to no such screening.122 At a median follow-up of 9 years, PSA screening showed a modest reduction in death from prostate cancer that would require screening of 1,410 men and treatment of 48 men to prevent one death from prostate cancer. The reduction in death from prostate cancer was apparent only in men age 55 to 69 years. The contemporaneous U.S.-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer screening trial randomized 76,693 men age 55 to 74 years to annual PSA testing for 6 years and annual DRE for 4 years versus no such screening.123 At a median follow-up of 7 years, no difference in mortality was noted between the arms. As of August 2008, the U.S. Preventive Services Task Force recommended against PSA screening in men age 75 years or older and concluded that current evidence is inadequate to make a PSA screening recommendation for men younger than the age of 75 years.124 The American Urological Association continues to recommend annual DRE and PSA screening tests for men older than 40 years of age if their life expectancy is >10 years.125
Radioimmunoassays for prostatic acid phosphatase (PAP) have a sensitivity of approximately 10% and a specificity of about 90% for malignant tumors118 and to a large extent have been superseded by PSA testing. Stamey et al.126 reported PSA and PAP measurements by radioimmunoassay in 2,200 serum samples from 699 patients, 378 of whom were known to have prostatic carcinoma. PSA was elevated in 122 of 127 patients with newly diagnosed prostatic carcinoma, whereas PAP was elevated in only 57 patients with cancer and correlated less closely with tumor volume than did PSA. PSA was increased in 86% and PAP in 14% of the patients with benign prostatic hyperplasia. After RP for cancer, PSA routinely declines to undetectable levels, with an associated half-life of 2.2 days. PAP, if initially elevated, normalizes within 24 hours after surgery. Several authors concluded that PSA is more sensitive than PAP and DRE in the detection of prostatic carcinoma and that PSA would be more useful in monitoring response and recurrence after therapy.126–128 A caveat is that both PSA and PAP may be elevated in benign prostatic hyperplasia. Hudson et al.127 reported that only 3% of 168 men with benign prostatic hyperplasia had PSA levels of >10 ng/mL, compared with 44% of 231 patients with prostatic carcinoma.
Several investigators129–131 reported no significant impact of DRE on the plasma levels of PSA or PAP in patients with various prostatic abnormalities in whom blood samples were collected before, immediately after, and 30 minutes after rectal examination of the prostate. Others132–134 detected a significant increase in PSA after DRE. Ornstein et al.134 noted an increase in both total and free PSA in 31% and 48% of men, respectively, at 1 hour after DRE. Matzkin et al.135 found no significant change in PSA levels after inserting a urethral catheter and maintaining it for several days. Yet, significant PSA elevation has been demonstrated after prostatic massage, transrectal ultrasonography (TRUS), prostate biopsy, and transurethral resection of the prostate (TURP).131,132,136,137 The kinetics of serum PSA elevation after DRE and needle biopsy were investigated in a Dutch study with few participants.138 Blood samples were taken at 1 and 30 minutes and 1, 3, 6, and 12 hours and then every 24 hours until 5 days had elapsed. The peak levels were between 30 and 60 minutes after DRE and returned to baseline 24 to 72 hours after the examination. There was a threefold increase in PSA after needle biopsy, and only two of seven patients had returned to their baseline PSA at 5 days. Studies reporting the effect of ejaculation on PSA also have contradictory results. Some authors found no effect at all,139,140 whereas others demonstrated that ejaculation increased PSA levels in 87% of 64 men evaluated with serial determinations (at 1, 6, 24, and 48 hours).141 A return to baseline was observed in 92% of subjects by 24 hours and in 97% by 48 hours.
Nadler et al.142 quantified causes of elevated PSA in 148 men with PSA of >4 ng/mL (a finding suspect for cancer) and multiple negative biopsies. They were compared with 64 men who had a suspect DRE, multiple negative biopsies, and PSA of ≤4 ng/mL. Acute or chronic inflammation of the prostate was more prevalent in the high-PSA group (63% vs. 27%; p = .0001). Patients with elevated PSA had significantly larger prostate volumes (median, 68 cm3) than those without PSA elevation (median, 32.5 cm3). Simultaneous regression analysis demonstrated that prostatic size accounted for 23%, inflammation for 7%, prostatic calculi for 3%, and nonisoechoic ultrasonographic lesions for 1% of the PSA serum variances.
The positive predictive value for PSA of >4 ng/mL ranges from 31% to 54%. A greater yield is observed when elevated PSA is coupled with positive ultrasonographic and rectal examinations (Tables 65.4A and 65.4B).143 The estimated rate of cancer detection by PSA screening ranges from 1.8% to 3.3%. The percentage of clinically localized tumors detected by PSA ranges from 81% to 97%. The percentage of pathologically localized tumors ranges from 36% to 91%, and is significantly higher when serial PSA screening is done.
An important issue is the clinical significance of small tumors detected by PSA testing. Epstein et al.89,144 identified a subset of patients with potentially biologically insignificant tumors among men with clinical stage T1c disease who underwent RP. On multivariate analysis, the best model predicting insignificant tumor was PSA density of <0.1 ng/mL per gram and no adverse pathologic findings on needle biopsy, or PSA density of 0.1 to 0.15 ng/mL per gram, with a low- to intermediate-grade cancer <3 mm found in only one needle biopsy core specimen. The positive predictive value of the model was 95%, with a negative predictive value of 66%.89 Dugan et al.145 offered a definition of clinically insignificant cancer as a tumor that gives rise to no more than 20 cm3 of cancerous tissue in the prostate by the time of expected death and a Gleason score of <4 in patients 40 years old, 5 in 50- to 59-year-old patients, 6 in 60- to 69-year-old patients, and 7 in 70- to 79-year-old patients. Using these definitions, a review of 337 prostatectomy specimens showed that, for cancer volume doubling times of 2, 3, 4, and 6 years, clinically insignificant cancer was identified in 1 (0.3%), 13 (3.9%), 25 (7.4%), and 49 (14.5%) of specimens, respectively. Humphrey et al.146 determined that in 11% to 30% of PSA-detected prostate cancers, the tumor volumes were <0.5 mL. Therefore, by these definitions, most men treated with RP (or radiation therapy) have clinically significant cancer.
The incidence of clinically unimportant cancers has been reported to be between 4% and 16%.89,101,147,148 Researchers at the Fred Hutchinson Cancer Center developed a computer model to estimate the rates of prostate cancer overdiagnosis because of PSA testing. Using the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry data as a comparison for their computer-generated incidence rates, they calculated overdiagnosis rates of 15% in Whites and 37% in Blacks. These men were predicted to have prostate cancer that would be detected only at autopsy.149 Conversely, an epidemiologic study randomizing men from Göteborg, Sweden, to PSA screening or a control group demonstrated that screening did not lead to overdiagnosis of prostate cancer; rather, most cancers detected at PSA-guided screening would eventually develop into clinical, frequently fatal, disease.150
Although PSA screening–detected cancers may be smaller, they may harbor aggressive disease. Investigators from the Netherlands studied 121 RP specimens from screened patients and found that screening-detected specimens were more likely to be pathologically organ-confined tumors and to have Gleason scores of <8.151 However, 60% of screening-detected tumors contained areas with high-grade cancer (Gleason pattern 4 or 5), and 50% had a Gleason score of 7, suggesting that most of these tumors are clinically important. Updated results from a study of expectant management of patients with nonpalpable prostate cancer believed to have small-volume disease showed a 6.5-year median survival free of intervention and a 33% intervention rate at a median of 2.2 years, with a median follow-up among the entire cohort of 2.7 years.152 Increased PSA density and decreased percentage free PSA correlated with progression of disease. Ninety-two percent of patients had curable disease at the time of their diagnosis of progression. These investigators concluded that observation with close follow-up may be a reasonable alternative for older men with a high likelihood of harboring small-volume prostate cancer.
Refinements of the PSA screening test have been introduced to increase the sensitivity and specificity of the test. It was anticipated that such approaches would be able to more readily find curable cancers in younger men and to avoid unnecessary biopsies of benign hypertrophic disease in older men. Unfortunately, none of these modifications listed in the following sections has proven reliable enough alone on which to base a treatment decision for the individual patient.
TABLE 65.4A LIKELIHOOD OF DETECTING PROSTATE CANCER ON TRANSRECTAL BIOPSY FOR 2,054 MEN AGE 40 TO 80 YEARS AS A FUNCTION OF SERUM PSA LEVEL, INDEPENDENT OF DRE RESULT
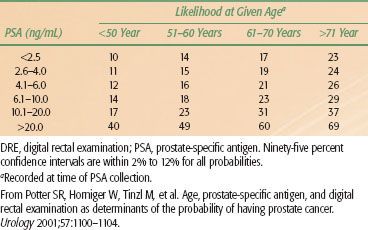
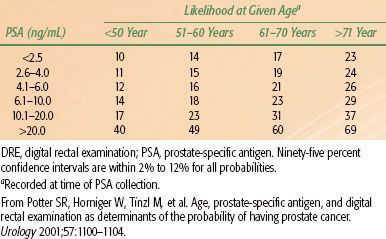
TABLE 65.4B LIKELIHOOD OF DETECTING PROSTATE CANCER ON TRANSRECTAL BIOPSY FOR 2,054 MEN AGE 40 TO 80 YEARS AS A FUNCTION OF PATIENT AGE, SERUM PSA LEVEL, AND DRE FINDINGS
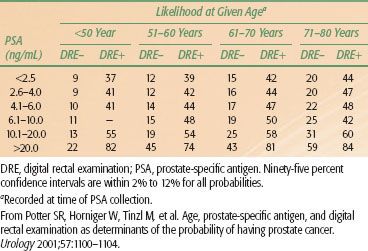
Prostate-Specific Antigen Density
PSA density relies on the fact that cancers produce less PSA per cell than nonmalignant prostatic tissues. It is calculated by dividing the serum PSA concentration by the volume of the prostate gland measured by TRUS. A higher PSA density is associated with malignancy.
Prostate-Specific Antigen Velocity
Another method is to obtain serial PSA measurements and calculate the rate of rise in PSA, or PSA velocity. A rate of rise of >0.75 ng/mL per year has been associated with a higher frequency of cancer. Two large retrospective analyses suggested that a >2.0 ng/mL increase in PSA in the year prior to diagnosis is correlated with greater prostate cancer–specific mortality following radiotherapy153 and RP154 for localized prostate cancer, respectively.
Free Prostate-Specific Antigen
Serum tests for the molecular forms of PSA (free vs. complexed vs. total) have been developed to discriminate between elevated PSA levels from benign prostatic hyperplasia versus cancer. This is based on the concept that PSA exists in serum in a complexed form bound to either α1-antichymotrypsin or α2-macroglobulin, two extracellular protease inhibitors. Bound to α1-antichymotrypsin or α2-macroglobulin, the enzyme is inactive but still detectable using conventional immunoassays. In a study of free PSA, complexed PSA, and total PSA (free + complexed), the complexed-to-total ratio was higher and free PSA lower in patients with prostate cancer relative to those with benign prostatic hyperplasia.155 Catalona et al.156 reported on 113 patients with PSA levels between 4.1 and 10 ng/mL (63 with histologically confirmed benign prostatic hyperplasia, 30 with prostate cancer and enlarged gland, and 20 with cancer and a normal-sized gland). The median percentage of free PSA was 9.2% for men with cancer and a normal-sized gland, 15.9% for those with cancer and an enlarged gland, and 18.8% for those with benign prostatic hyperplasia (p < .001). Men with prostate cancer and either a normal or an enlarged gland had a significantly lower percentage of free PSA than men with benign prostatic hyperplasia only. At Washington University, a ratio of free to total PSA of ≤0.2 was most likely associated with prostate cancer and with higher percentages with benign prostatic hypertrophy. A ratio of ≤0.15 was associated with a higher Gleason score and poorer prognosis.157
Oesterling et al.158 analyzed free, complexed, and total PSA in 422 healthy men aged 40 to 79 years. The respective recommended age-specific reference ranges (95th percentile) for the three forms were 0.5, 1, and 1 ng/mL for men aged 40 to 49 years; 0.7, 1.5, and 3 ng/mL for men aged 50 to 59 years; 1, 2, and 4 ng/mL for men aged 60 to 69 years; and 1.2, 3, and 5.5 ng/mL for men aged 70 to 79 years. Similar observations were made by investigators at Johns Hopkins.159
Reverse Transcriptase–Polymerase Chain Reaction Assay
Recent developments include using molecular biologic methods, particularly reverse transcriptase–PCR (RT-PCR), to measure markers by detecting low levels of messenger RNA (mRNA) for PSA and prostate-specific membrane antigen (PSMA) expressed by circulating metastatic prostate cancer cells.160–164 The assay is highly specific because the only cells expressing PSA in the peripheral blood are circulating prostate cancer cells. However, there is a wide range of sensitivities of detection of PSA-expressing cells in the peripheral blood reported in the literature.165
Katz et al.,166 in 94 patients on whom RT-PCR assay for PSA mRNA was performed, reported an enhanced reaction in 26 (72%) of 36 patients who had extraprostatic tumor at the time of surgery. The test was negative in 51 (88%) of 58 patients with organ-confined disease. Six months after surgery, an increased PSA level was noted in 19% and 2% of the two groups, respectively. This bioassay may have significant staging value in patients who are candidates for RP.
Cama et al.160 noted that, in contrast to the RT-PCR assay for PSA, the assay for PSMA did not correlate with pathologic stage of prostate cancer.
Oefelein et al.,167 using RT-PCR for PSA, identified positive cells in 20 (91%) of 22 operative field samples, and 4 (25%) of 16 had evidence of intraoperative hematogenous dissemination (p = .046). Their results suggest that tumor cell spillage and, less frequently, hematogenous dissemination may be associated with operative manipulation of the prostate during RP and may potentially represent the mechanism of failure after this treatment.
Israeli et al.,168 using the PCR assay, also reported circulating prostatic tumor cells in 2 (6.7%) of 30 men. However, prostate-specific membrane primer assay demonstrated tumor cells in 19 (63%) of 30 patients. All 16 negative control subjects had negative PSA and PSMA PCR results. Using PSA mRNA as a marker for prostatic epithelial cells, Seiden et al.,169 noted that 5 of 65 patients with clinically localized carcinoma of the prostate had PSA mRNA–detectable cells by transcription and PCR. On the other hand, 10 of 20 patients with hormone-refractory and progressive prostate cancer also demonstrated the same increased frequency of PSA mRNA–detectable cells.
Overall, most studies report a 0% PSA rate by RT-PCR in negative control cases, whereas the positive rate in the metastatic group ranges between 31% and 88%.164 However, 25% of men with localized prostate cancer who underwent RP and had specimen-confined disease had a positive PCR PSA assay.166 These men would be denied surgery if it was concluded that circulating prostate cancer cells are synonymous with incurable disease. A new approach is to use a combination of primers to improve the overall staging accuracy of RT-PCR. Preliminary work from the Cleveland Clinic suggests that combining RT-PCR for PSA and PSMA may improve the staging accuracy.170 Until the significance of a positive PCR assay is determined with long-term follow-up, RT-PCR remains experimental and should not change treatment recommendations.
Staging Workup
Patients with localized prostatic carcinoma are frequently asymptomatic; the diagnosis is often made with a screening PSA test. In the pre-PSA era, asymptomatic patients were diagnosed on the basis of palpating a hard nodule on DRE. Patients with locally advanced tumors have presented with bladder outlet obstructive symptoms such as urinary hesitancy, decreased force of the urinary stream, and postvoid dribbling as the tumor impinges on the membranous urethra. Chronic obstruction and bladder distention can lead to decreased compliance of the detrusor muscle that is manifested by symptoms of urinary frequency, urgency, and nocturia. Very early–stage disease (T1a or T1b) may occasionally be diagnosed at TURP for symptoms of bladder outlet obstruction caused by benign prostatic hyperplasia. With local invasion into the urethra or ejaculatory ducts, patients may experience hematuria or hematospermia. As the disease penetrates the capsule of the prostate, there may be invasion into the neurovascular bundles that course along the lateral aspects of the prostate, leading to erectile dysfunction. Disseminated disease frequently manifests as bone pain from distant osseous metastases.
A complete clinical history and a general physical examination including DRE are mandatory. The DRE is best performed with a well-lubricated glove; the patient may be standing and bent over at the waist with his elbows resting comfortably on a firm surface or in the lateral decubitus position on the examining table. The examiner should note the size of the gland, its overall consistency, and the presence of any firm areas. A typical neoplastic nodule of prostatic carcinoma is extremely firm, often not elevated above the surface of the gland, but surrounded by compressible prostatic tissue. The examiner should determine whether the lateral sulci are involved by tumor and also the degree of spread superiorly. In most patients the seminal vesicles cannot be palpated as discrete structures, and the finding of a firm area extending above the prostate suggests that the seminal vesicles are involved by malignancy. Only approximately 50% of prostatic nodules found on DRE are confirmed to be malignant on biopsy.79 The American Joint Committee on Cancer TNM Staging System for prostate cancer is shown in Table 65.5.
An abnormal DRE result, a consistently elevated PSA, or a combination of the two warrants a biopsy to establish a pathologic diagnosis. A TRUS-guided needle biopsy is the most common method for obtaining representative samples of the prostatic tissue. Ten to 18 cores are taken, including cores from the base, mid, and apex bilaterally and additional cores from the midline and lateral peripheral zone. If clinically indicated by obstructive symptoms, a separate biopsy of the TZ is taken. The pathology report frequently includes the length of each core and the length of each core that contains tumor.
Once the tissue diagnosis of prostate cancer is ascertained, the patient should undergo a staging workup including laboratory data such as a baseline PSA, complete blood count, and testosterone level. The standard tests required in the evaluation of patients with prostatic carcinoma are listed in Table 65.6. Although a chest radiograph is recommended, a study of 236 patients undergoing RP showed abnormal findings in only 28 (11.9%), mostly related to cardiac or pulmonary problems or arterial hypertension; one primary lung cancer was found.171 According to the American College of Radiology appropriateness criteria, a chest radiograph should be performed as part of the initial staging only with suspected metastatic disease.172
Imaging Studies
Diagnostic imaging studies have become an essential aspect of pretreatment evaluation and treatment selection. New techniques have allowed for more precise assessments of tumor location, volume, and extent, as well as biologic activity. As a result, clinical staging can be used more accurately as a prognostic factor for defining treatment options.
TABLE 65.5 AMERICAN JOINT COMMITTEE ON CANCER 2010 TNM STAGING SYSTEM FOR PROSTATE CANCER





