Chapter 24 Low-Grade Gliomas
Epidemiology and Etiology
In 2010 in the United States, it is estimated that 22,020 CNS tumors will be diagnosed,1 of which approximately 19,000 will arise in the brain.2 Of these, approximately 2000 will be low-grade gliomas.3
Etiologic factors for low-grade gliomas are largely unknown. Low-grade astrocytomas have been associated with von Recklinghausen’s disease (type 1 neurofibromatosis) and type 2 neurofibromatosis.2 In addition, a direct link has been made between subependymal giant cell astrocytoma, an uncommon pathologic type of low-grade astrocytoma, and tuberous sclerosis.4
Pathology
Pathologic Classification
Historically, low-grade gliomas have been considered a homogeneous group of neoplasms associated with a benign or favorable natural history.5 Actually, they are a diverse group of tumors found throughout the CNS, the outcome of which depends on a number of anatomic, pathologic, and treatment factors.
Table 24-1 summarizes the current World Health Organization (WHO) classification of primary CNS tumors as it applies to low-grade gliomas.6 Astrocytic tumors can be classified broadly into three groups. The diffusely infiltrative low-grade astrocytomas (WHO grade II) are the most common and include the fibrillary, protoplasmic, and gemistocytic types (hereafter called diffuse astrocytomas). They represent 70% of low-grade cerebral astrocytomas.7 Diffuse astrocytomas are usually poorly circumscribed and are capable of undergoing anaplastic transformation with an incidence as high as 79%.8 The pilocytic astrocytomas (WHO grade I), which comprise nearly all of the remainder of the cerebral astrocytomas, tend to be better circumscribed and rarely transform.9 Although pilocytic astrocytomas occur more commonly in the cerebellum of children (juvenile pilocytic astrocytoma), they also occur in the cerebral hemispheres and near the optic tracts.9 Remaining are the uncommon low-grade glioma variants, including the pleomorphic xanthoastrocytomas, subependymal giant cell astrocytomas, and others, which are briefly discussed later in the chapter.
TABLE 24-1 Low-Grade Gliomas* Included in World Health Organization (WHO) Classification of Gliomas
| Type | WHO Grade |
|---|---|
| Astrocytic Tumors | |
| Subependymal giant cell astrocytomas | I |
| Pilocytic astrocytomas | I |
| Pleomorphic xanthoastrocytomas | II |
| Pilomyxoid astrocytoma | II |
| Diffuse astrocytoma | II |
| Oligodendroglial Tumors | |
| Oligodendrogliomas | II |
| Oligoastrocytic Tumors | |
| Oligoastrocytomas | II |
Louis DN, Ohgaki H, Wiestler OD, et al: The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol 114(2):97-109, 2007.
Grading Systems
The concept of dividing astrocytomas into discrete grades associated with a distinct clinical prognosis dates back to the mid-1920s and early 1930s to the work of Bailey and Cushing, who recognized a subset of astrocytomas that had a more favorable outcome than glioblastoma.10 There have been many grading systems (e.g., Kernohan, St. Anne-Mayo, and Ringertz systems) in the past, and most of these grading systems share an assessment of nuclear abnormalities, mitoses, endothelial proliferation, and necrosis, but the most widely used and accepted grading system today is the WHO system.6 WHO grade I lesions have low proliferative potential, with the possibility of cure following surgery alone. Grade II neoplasms are infiltrative, often recur, and tend to progress to higher grades of malignancy (e.g., grade II astrocytoma transforms to grade III anaplastic astrocytoma) despite low-level proliferative activity. All grading schemes are limited by their need to separate gliomas artificially into three or four groups, when in actuality they exist along a biologic continuum. For the present, these proven but awkward methods retain their utility in both therapeutic decision making and prognostication. It is anticipated that future reiterations of the WHO grading system will incorporate imaging and genetic testing.
Biologic Characteristics and Molecular Biology
Studies of Proliferation
Tumor proliferation can be assessed with Ki-67 labeling. A large review on Ki-67 labeling revealed increasing values of Ki-67/MIB-1 labeling index with increasing grade of malignancy.11 The MIB-1 labeling index differentiates diffuse astrocytomas (WHO grade II) from anaplastic astrocytomas (WHO grade III) and glioblastoma multiforme (GM) tumors. Similar findings have been reported in oligodendrogliomas.12 Although the MIB-1 labeling index is a clear prognostic factor in low-grade glioma, the absence of agreed upon low versus high values and interobserver variation between various institutions prevents its routine use.
Genetic and Other Molecular Studies
About 50% to 60% of astrocytomas have TP53 mutations, and this figure may be even higher in gemistocytic astrocytoma.13,14 In contrast, low-grade oligodendrogliomas typically have combined loss of 1p/19q, and the genotype is actually an unbalanced t(1;19)(p10;q10) translocation.15 TP53 mutations and 1p/19q co-deletions are mutually exclusive, and most mixed oligoastrocytomas carry either the typical oligodendroglial 1p/19q co-deletion or a TP53 mutation, suggestive of an astrocytic lineage.16 Therefore, at the molecular level, mixed oligoastrocytomas are not true mixed tumors but are of either astrocytic or oligodendroglial lineage.
The recently identified IDH1 mutations (an enzyme that participates in the citric acid cycle) occur in 70% to 80% of low-grade gliomas, surprisingly both in tumors with TP53 mutations and in tumors with the 1p/19q loss.14,17 The current data and the absence of the acquisition of IDH1 mutations over time suggest that the IDH1 mutation is an early event in the tumorigenesis of IDH1-mutated tumors18; as an example, in one series IDH1 mutations occurred in only 3% of primary glioblastomas but in 50% of secondary glioblastomas.17
O6-Methylguanine DNA methyltransferase (MGMT) is a DNA-repair enzyme that removes alkyl groups from the O6 position of guanine in DNA and therefore repairs damage induced by alkylating agents (e.g., BCNU, temozolomide). Methylation of the O6-methylguanine-methyltransferase promoter has been observed in 40% to 95% of low-grade tumors; this large range in methylation of MGMT may in part be explained by differences in technique and laboratory procedures.19–21 Of note, there is significant interobserver variation in the assessment of MGMT expression with immunohistochemical testing, which raises questions regarding the usefulness of the assay.
The PI3K/mTOR pathway is activated in most adult low-grade gliomas, with methylation of the promoter region of the PTEN gene in one third of patients.22 One study observed that p14(ARF) hypermethylation and MGMT hypermethylation constitute distinct molecular pathways of astrocytoma progression, with more frequent progression in p14(ARF) hypermethylation cases.23
Prognostic and Predictive Molecular Factors
In astrocytoma and mixed oligoastrocytoma, the presence of TP53 mutations but not p53 protein overexpression was correlated with survival.13 In addition, the activation of the PI3K/mTOR pathway was found to predict patient survival.22 Presently there is limited information on the clinical impact of IDH1 and IDH2 mutations. In one study the presence of IDH1 mutations had a major favorable prognostic impact in low-grade astrocytomas but did not affect outcome with temozolomide treatment.14
In oligodendrogliomas, the presence of the 1p/19q co-deletion confers a favorable prognosis, with slower growth rates, compared with non–co-deleted low-grade gliomas.24,25 In a study of 1p/19q status in 98 low-grade glioma patients enrolled in two North Central Cancer Treatment Group (NCCTG) protocols, the 1p/19q co-deletion was seen in 57%, 32%, and 0% of oligodendrogliomas, oligoastrocytomas, and astrocytomas, respectively.15 In oligodendroglioma patients, the median overall survival (OS) time and 5-year OS were 13 years and 96% in patients with 1p/19q co-deleted tumors versus 10.8 years and 70% in those without co-deletion. For oligoastrocytomas, the median OS and 5-year OS were 11 years and 90% for 1p/19q co-deleted tumors and 8.3 years and 62% for tumors without the 1p/19q co-deletion. Several studies noted improved outcome with chemotherapy of 1p/19q co-deleted tumors as opposed to 1p/19q intact (oligodendroglial) tumors.26
MGMT promoter methylation results in epigenetic silencing and thereby decreased MGMT expression, which is associated with longer survival in patients with glioblastoma who receive alkylating agents but has also been related to improved PFS in irradiated grade III tumors.27 Because of such findings, it is uncertain whether MGMT promoter methylation is prognostic or predictive of outcome to treatment. In low-grade gliomas treated with neoadjuvant temozolomide, the presence of a methylated MGMT promoter was found to be a favorable predictor of progression-free survival as compared with unmethylated tumors.19 Patients with methylated MGMT promoter also had longer OS in an uncontrolled trial of dose-dense temozolomide in recurrent low-grade glioma.28 However,another study found the presence of MGMT methylation to be associated with a shorter PFS time.20 Nonetheless, MGMT methylation predicted an increased sensitivity to alkylating chemotherapeutic agents.
Clinical Manifestations and Patient Evaluation
In this section, information is reviewed on the clinical presentation and imaging of supratentorial low-grade gliomas based on the literature.29
Clinical Presentation
The symptoms of low-grade gliomas are shown in Table 24-2. The most common symptom is seizure, occurring in two thirds of patients. Focal seizures are more common than generalized ones. Generalized tonic-clonic, simple partial, and complex partial seizures occur in 43%, 23%, and 34% of patients, respectively. Headache and weakness occur in approximately one third of patients. The remaining symptoms occur in 15% or fewer patients. The median duration from onset of symptoms to diagnosis is between 6 and 17 months, with a range of 1 day to 17 years.
TABLE 24-2 Symptoms of Low-Grade Gliomas
| Symptom | Frequency (%) Mean | Range |
|---|---|---|
| Seizure | 65 | 30-92 |
| Headache | 36 | 5-48 |
| Weakness | 30 | 6-53 |
| Visual loss or change | 15 | 4-16 |
| Personality change | 14 | 5-16 |
| Focal symptom, NOS | 13 | 6-20 |
| Language dysfunction | 13 | 4-17 |
| Altered sensation | 10 | 8-11 |
| Nausea and vomiting | 10 | 4-14 |
| Altered mental status | 9 | 7-12 |
| Altered consciousness | 8 | 4-20 |
| Cranial neuropathy, NOS | 7 | — |
NOS, not otherwise specified; —, data reported in only one series.
Data from Levin VA, Gutin PH, Leibel S: Neoplasms of the central nervous system. In DeVita V, Hellman J, Rosenberg S (eds): Cancer: Principles and Practice of Oncology, 4th ed. Philadelphia, JB Lippincott, 1993.
Prognosis by Clinical Presentation
Age, gender, symptoms, signs, and neurologic function have all been significantly associated with survival in univariate and multivariate analyses from a number of retrospective studies in the literature. Both preoperative and postoperative neurologic function and performance status are correlated with outcome.30 Brown and colleagues30 conducted an analysis of baseline (before radiation therapy) Mini-Mental State Examination (MMSE) scores collected in patients with low-grade glioma on a prospective, intergroup clinical trial and found that the presence of an abnormal baseline MMSE score (26 or less) was a strong predictor of worse outcome. Patients with an abnormal baseline MMSE score had worse 5-year PFS (27% vs. 60%) and OS (31% vs. 76%) compared with those with a normal score.
The most powerful predictor of survival in patients with low-grade glioma is age, with much better survival rates in pediatric patients compared with adults. Table 24-3 shows the survival rate of children with low-grade astrocytoma. In a phase II trial of 78 pediatric low-grade glioma patients treated with radiotherapy (54 Gy), the 5- and 10-year OS were 99% and 96%, respectively.31 For adult patients with low-grade astrocytoma, there is also an association between age and survival. Those less than 40 years of age have a better survival than older patients.32
Of all the neurologic symptoms associated with low-grade astrocytoma, seizures, particularly when preceding the diagnosis by 6 months or more and in the absence of other neurologic symptoms, are associated with a better prognosis. In one series, the 5-year OS was 47% in patients presenting with seizures, 33% in those with headaches, 20% in those with altered mental status, and 0% when stupor was the presenting symptom.33 In another series, the 5-year OS was 64% in patients with low-grade glioma who had seizures versus 14% in those without seizures.34
Patients who present with a history of chronic epilepsy resulting from an underlying cerebral neoplasm are frequently found to have a low-grade astrocytoma. These patients are usually children or young adults (30 years of age or younger) whose seizure disorder and underlying tumor are often cured by a complete resection.35
Imaging of Low-Grade Gliomas
Table 24-4 shows the imaging characteristics of supratentorial low-grade astrocytomas based on multiple series in the literature.7 On computed tomography (CT), the typical low-grade astrocytoma is lobar in location, involves the frontal or temporal lobes, is larger than 5 cm in diameter, and is nonenhancing. Because of the infiltrative nature of low-grade astrocytomas, the CT appearance of a typical nonenhancing tumor is a poorly defined area of low attenuation (Fig. 24-1A).
TABLE 24-4 Imaging Characteristics of Supratentorial Low-Grade Astrocytoma
| Characteristics | Frequency (%) Mean | Range |
|---|---|---|
| Location | ||
| Lobar | 80 | 50-90 |
| Deep/midline* | 20 | 4-40 |
| Right | 50 | 42-55 |
| Left | 50 | 41-60 |
| Bilateral | 5 | 1-11 |
| Hemispheric† | 0.5 | — |
| Lobar Sites | ||
| Single | 84 | — |
| Multiple | 16 | 13-38 |
| Frontal | 44 | 38-65 |
| Temporal | 37 | 8-61 |
| Parietal | 18 | 5-40 |
| Occipital | 3 | 0-10 |
| Other | ||
| Enhancement | 34 | 22-58 |
| Size ≥5 cm | 66 | 21-88 |
| Calcification | 10 | 3-18 |
| Cyst | 15 | 6-29 |
| Mass effect‡ | — | 12-84 |
* Deep/midline sites include thalamus, basal ganglia, hypothalamus, third ventricle, and corpus callosum.
† Only one series presented data on hemispheric involvement.89
‡ Only two series presented data on mass effect.86,90
From Shaw EG, Scheithauer BW, Dinapoli PR: Low-grade hemispheric astrocytomas. In Black PM, Loeffler JS (eds): Cancer of the Nervous System. Cambridge, Blackwell Scientific, 1997.
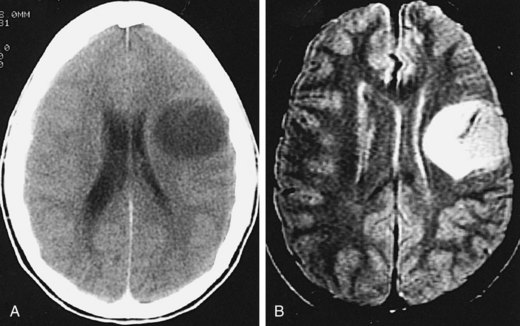
MRI (Fig. 24-1B) provides superior visualization. FLAIR (fluid-attenuated inversion recovery) and both T2- and T1-weighted images (without and with contrast) provide anatomic detail that is useful in defining the extent of the tumor and in planning for surgical and radiation therapy. An understanding of these imaging-based anatomic-histopathologic relationships is important for both the neurosurgeon and the radiation oncologist. Removal of the poorly defined area of low attenuation (increased T2 or FLAIR signal) in a functionally intact area of brain (Fig. 24-2) may lead to significant neurologic morbidity.
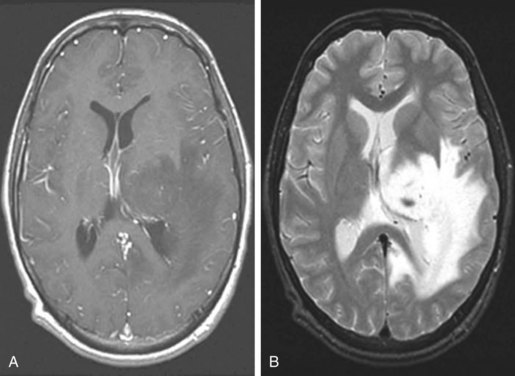
The radiographic features of low-grade gliomas have been correlated with prognosis in a number of retrospective series. Although the presence of contrast enhancement on a CT or MR scan in nonpilocytic low-grade gliomas would seemingly be associated with a worse prognosis because of presumed higher-grade elements or malignant transformation, data from prospective trials have not found the presence of contrast enhancement to be a predictor of outcome.36,37 This may be related to the type of enhancement. Nodular enhancement as opposed to faint, patchy enhancement appears to be related to decreased survival times.38 Other imaging findings, such as tumor size greater than 5 or 6 cm, or tumor crossing the midline (Fig. 24-3), have been consistently associated with worse outcome.32,36
Pignatti and colleagues32 developed a prognostic scoring system using imaging, patient, and tumor characteristics derived from the databases of two large phase III adult low-grade glioma trials (European Organization for Research and Treatment of Cancer [EORTC] trials 22844 and 22845). By means of Cox regression analysis, they identified and validated the following negative risk factors: age 40 years or older, astrocytoma histology subtype, largest diameter of the tumor of 6 cm or more, tumor crossing the midline, and presence of neurologic deficit before surgery. The total number of unfavorable factors present was found to be useful in predicting outcome. The presence of two of these factors or fewer identified a low-risk group (median survival, 7.7 years), whereas three risk factors or more identified a high-risk group (median survival, 3.2 years).
Primary Therapy
Observation
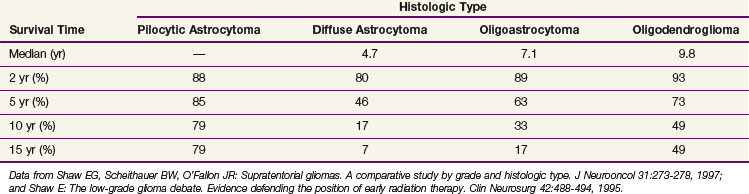
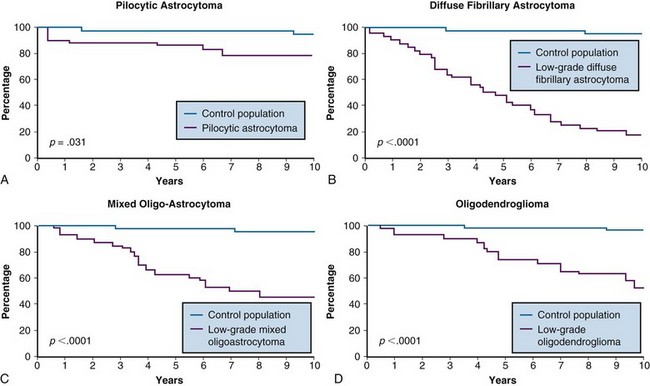
Despite the favorable survival rates observed in certain subsets of patients with low-grade gliomas, the natural history of all pathologic types of supratentorial low-grade gliomas, including the pilocytic astrocytomas (WHO I), diffuse astrocytomas, oligoastrocytomas, and oligodendrogliomas (WHO II), is significantly worse than that of an age- and sex-matched control population, for which the expected survival rate is greater than 95%39 (Table 24-5 and Fig. 24-4). Based on this observation, some have argued that all such patients should undergo maximal safe surgical resection followed by postoperative radiation therapy, although a survival benefit for treatment, with either aggressive surgery or radiation therapy has not been demonstrated in prospective clinical trials.
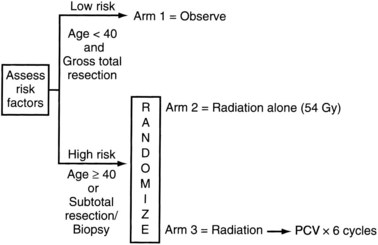
The Radiation Therapy Oncology Group (RTOG) phase II portion of protocol 9802 (Fig. 24-5) prospectively observed 111 low-risk (age younger than 40 years and neurosurgically defined gross total resection) low-grade glioma patients.37 In this “low-risk” population, the 5-year OS was 93%. Astrocytoma histology, residual tumor of 1 cm or more according to MR imaging, and preoperative tumor diameter of 4 cm or more were found to be predictive of a poorer PFS. In patients with all three unfavorable prognostic factors, the 2- and 5-year rates of PFS were 60% and 13%, respectively. In patients with none of the three unfavorable prognostic factors, the 2- and 5-year rates of PFS were 100% and 70%, respectively. These data suggest that observation is a reasonable strategy for the most favorable subset (<1 cm residual tumor, preoperative tumor diameter <4 cm, and oligodendroglioma histology) of younger patients after a gross total resection (GTR).
An EORTC phase III trial (22845) of 311 adults with supratentorial low-grade gliomas randomized patients to either observation with radiation therapy at progression (which should be considered a delayed radiotherapy arm because two-thirds of the patients in the observation group received radiotherapy at the time of progression) or initial radiation therapy using 54 Gy to localized treatment fields.40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree