INTRODUCTION
Over the last four decades, substantial improvements in treatment effectiveness for childhood and adult cancers have resulted in cure or increased survival for these populations. Over 80% of all patients diagnosed with cancer before the age of 20 years will be surviving at 5 years. As a consequence of both improved survival rates and increasing incidence of childhood cancer, the number of long-term survivors of childhood cancer in the United States is rapidly increasing. An estimated 320,000 or more childhood cancer survivors are living in the United States, and at least 75% of these survivors are now adults. Of these, 24% have survived more than 30 years (1,2). These individuals are living long enough to demonstrate the lifelong consequences of the cancer and treatments (3,4). The numbers of adult cancer survivors are growing as well, with an estimated 18 million adult survivors in the United States by 2022 (Fig. 60-1).
FIGURE 60-1
Estimated and projected number of cancer survivors in the United States. Reproduced with permission from de Moor JS, Mariotto AB, Parry C, et al: Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care, Cancer Epidemiol Biomarkers Prev 2013 Apr;22(4):561-570.
In 2006, the Institute of Medicine published From Cancer Patient to Cancer Survivor: Lost in Transition (Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies; 2006). This seminal report defined the “essential” components of survivorship care as (1) prevention of recurrent and new cancers; (2) surveillance for recurrence, secondary cancers, and medical effects of treatment; (3) intervention for the sequelae of cancer and its treatment; and (4) coordination of care between specialists and primary care providers. Other chapters address disease-specific surveillance; this chapter expands on the first three components of the report.
SCREENING FOR SECONDARY MALIGNANCIES
Patients who have been treated for a cancer are at higher risk for a second primary cancer or recurrence of their primary tumor, most particularly at the time of their transition from active cancer treatment to survivorship. In fact, second- and higher-order cancers accounted for as many as 16% of incident cancers in the SEER (Surveillance, Epidemiology, and End Results Program) database as of 2003 (5). The reasons for this are varied based on malignancy and include factors related to previous therapy (radiation, chemotherapy, hormonal therapies); previous or ongoing exposure to carcinogens; predisposing conditions (tobacco, alcohol, sun exposure, dietary influences, immunologic dysfunction); and familial genetic syndromes, including the BRCA1 and BRCA2 breast cancer syndromes, Bloom’s syndrome, Cowden’s syndrome, Li-Fraumeni, Lynch syndrome, and others (6). The confluence of these factors, which are increased in their incidence in the population of cancer survivors, necessitates a careful approach to screening for second malignancies. In some cases, this entails increased surveillance for patients who have known risk factors related to their previous cancer or its treatment. For others, the cancer survivorship care provider must pay close attention to the best-practice screening guidelines that already exist for the greater population at large.
In survivors of childhood cancer, these issues are magnified. As childhood cancer survivors progress through adulthood, the risk of subsequent malignant neoplasms (SMNs) increases. Among 14,359 individuals who were 5-year survivors of childhood cancer at median of 30 years (range 5-56 years) the cumulative incidence was 20.5% at 30 years for subsequent neoplasm, representing a sixfold increased incidence compared to the age-adjusted general population (standardized incidence ratio, SIR) (7). The most frequent subsequent malignancies were nonmelanoma skin cancer and breast cancer. Patients treated in childhood for Hodgkin’s lymphoma and Ewing sarcoma had the highest risk (SIR 8.7 and 8.5, respectively). While the majority of SMNs are diagnosed within the first 10 years, they can occur up to 30 years after the original cancer diagnosis, with secondary leukemia having shorter latency than solid tumors (Table 60-1). Of solid tumors, the highest risks were observed for bone cancer (SIR 19), thyroid cancer (SIR 10.9), breast cancer (SIR 9.8), central nervous system (CNS) malignancies (SIR 10.4), and soft tissue sarcoma (SIR 8.1). In addition, survivors are at increased risk for other cancers, such as head and neck, kidney, bladder, lung, gastrointestinal and colon, and genitourinary cancers.
| Second Malignant Neoplasm | Risk Factors | Screening Recommendations |
|---|---|---|
| Skin cancer | 10%-20% of patients; increased risk in irradiated skin | Annual skin exams by dermatologist; close monitoring of irradiated skin and palms and soles |
| Breast cancer | Risk in women younger than 30 years is elevated 5 to 54 times depending on radiation dose to thorax | Yearly mammograms or magnetic resonance imaging of breasts beginning 8 years after radiation or age 25 years, whichever occurs later, for women who had chest radiation |
| Thyroid cancer | Increased risk with radiation to head, neck, or chest | Annual history and physical examination; free thyroxine, thyroid-stimulating hormone tested yearly; thyroid ultrasound every 3-4 years after treatment completion or sooner if nodule is found |
| Leukemia (acute myeloid leukemia/myelodysplastic syndrome) | Increased risk with exposure to alkylating agents, topoisomerase inhibitors | Annual history and physical examination, including complete blood cell count with differential and platelet count (highest risk first 5 years after exposure) |
| Brain tumors | Increased risk with cranial radiation; the younger the age at primary diagnosis, the greater the risk; received cranial radiation for brain tumor, acute lymphoblastic leukemia, some head and neck sarcomas | Latency period 9 to 10 years after radiation; monitor with annual history and physical examination, including yearly neurological exam (more often if indicated by examination or symptoms) |
| Other carcinomas | Can occur in patients who have or have not undergone radiation therapy | Latency period 5 to 30 years, median 15 years; yearly history and physical examination; if abdominal radiation: colon cancer screening with colonoscopy every 10 years beginning 15 years after completion of treatment or at age 35 years, whichever is later |
The increased risk of second malignancies is particularly well described in survivors of Hodgkin’s lymphoma (8,9,10,11). Radiation therapy, particularly when delivered in adolescence, is known to be a predisposing factor for a host of second cancers in the radiation field, including cancers of the skin, breast, lung, and connective tissue (8). Moreover, there is evidence to suggest that receiving chemotherapy in addition to radiation may increase the risk of subsequent development of solid tumors. Thus, it is not surprising that the risk of acute myeloid leukemia is also increased, likely due to both radiation exposure and the use of alkylating agents during treatment (11).
The recognition that patients who have received mantle radiation have a higher risk of malignancy has led to the tailoring of screening recommendations, particularly those regarding breast cancer, to address this increased susceptibility (12). While formal guidelines for screening for cancer at sites other than the breasts in the involved fields do not exist, a key function of interval updates to history and physical exam during survivorship care is to focus on these important possible late complications of cancer treatment.
Like survivors of Hodgkin’s lymphomas, there is significant literature regarding the importance of second cancers in patients with primary head and neck neoplasms (13,14). The human papilloma virus (HPV) is an important causative agent of oral and oropharyngeal cancers and also anal, vaginal, and cervical cancers. That said, there is little literature regarding synchronous or metachronous primary tumors of the anus or uterine cervix in patients with oral index tumors. Perhaps more important, tobacco use is a major risk factor for this category of cancers, as well as for cancers of the lung, bladder, and other sites. Consequently, as many as a third of deaths in patients with head and neck cancers are attributable to second primary cancers, a larger number than those that die from distant metastases from the first tumor. In a recent analysis based on SEER data, the incidence and type of second cancers varied based on the site of primary tumor. Patients with index oral and oropharyngeal cancers had more second head and neck tumors, while patients with hypopharyngeal and laryngeal cancers had more lung cancers as their second primary, likely reflecting the diminished importance of HPV infection and increased contribution of tobacco exposure as a cause of cancer at these sites.
While the volume of literature regarding second tumors in survivors of other cancers varies, the survivorship care provider must understand that any survivor of cancer or cancer treatment may be at risk. Like primary tumors, occurrence of second cancers is due to a confluence of environmental exposures, behavioral influences, and genetic milieu.
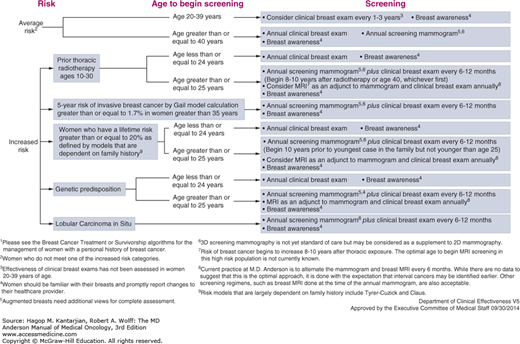
The most important factor modifying breast cancer screening (Fig. 60-2) in cancer survivors is a history of radiation exposure to the chest. Of women who received radiation therapy involving the breast during childhood and adolescence, 13% to 20% will go on to develop a breast malignancy by the age of 40 to 45 years. This risk is most pronounced for women who received 20 Gy or more of radiation to breast tissue, but the increased risk is measurable even among women who received 1 to 9 Gy. International consensus guidelines recommend starting screening at age 25 or 8 years after treatment, whichever comes last, for women who received 20 Gy or more of radiation, with individualization of the screening approach for women who received lower doses of radiation. Our approach at MD Anderson involves starting at age 25 or 8 to 10 years after exposure to radiation, whichever comes first, and we alternate annual mammograms and annual magnetic resonance imaging (MRI) every 6 months, with clinical breast exam done annually. While we acknowledge that data to support the use of MRI as the optimal approach in these patients is still in evolution, this modality has the added benefit of sparing additional radiation exposure while providing excellent imaging detail. Thus, the use of MRI in breast cancer screening has greatly increased over the last decade, particularly in the pool of high-risk women (15).
Similarly, for those patients who are diagnosed with predisposing familial syndromes during the workup for their breast cancers, we recommend annual MRI and mammograms, alternating every 6 months, with clinical breast exams every 6 to 12 months. The recommendations are similar for women with a lifetime breast cancer risk of 20% or more based on models that take into account family history. While screening recommendations for other survivors of breast cancer without identified predispositions do not materially differ from those for the general population, it is acknowledged that these women represent a higher-risk pool, and as such, adherence to screening regimens is of increased importance.
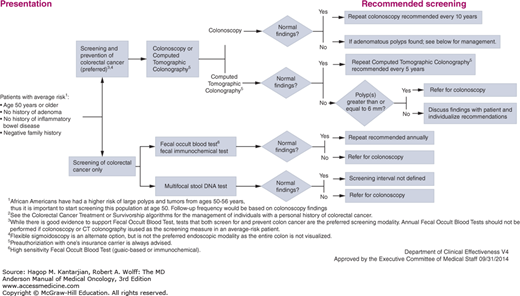
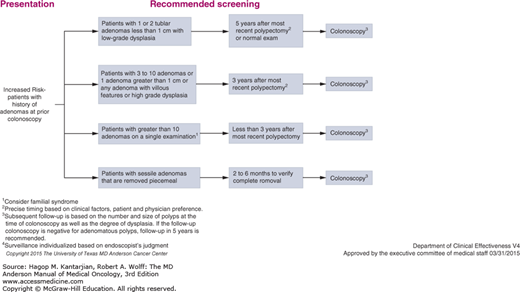
Colon cancer screening is also of increased importance among cancer survivors. There are several familial syndromes that predispose to both primary and second colon cancers, including the Lynch syndrome and familial adenomatous polyposis (FAP). In addition, environmental, dietary, and immunologic factors may contribute to an increased risk of second colonic primary in patients with a previous history of colorectal cancer or polyps. Thus, patients with a history of colon cancer require increased frequency of screening for second colorectal primary tumors indefinitely. In the MD Anderson colorectal screening guidelines, we suggest screening colonoscopy every 5 years in long-term survivors. For patients with adenomas at the time of colonoscopy, more frequent screening is recommended (Fig. 60-3).
For patients with a diagnosis of Lynch syndrome, some of whom may have a history of gynecologic or other solid tumors, colorectal cancer screening is recommended starting at age 25 or 10 years prior to the earliest colorectal cancer in the immediate family, with colonoscopies every 1 to 2 years thereafter.
The decreased incidence of cervical cancer–related mortality in the United States is related primarily due to widespread screening for premalignant lesions by Papanicolaou testing. Screening for cervical cancer is logically important in survivors of HPV-associated malignancies, including cervical, anal, and oral cancers as these patients likely have a history of high-risk HPV exposures, in addition to possible immunocompromised or other risk factors. In addition, tobacco exposure is an important risk factor that may predispose survivors of other tobacco-related tumors to cervical cancer. Screening for this disease may be particularly important in survivors of adolescent and young adult cancers. In a recent study based on SEER data, it was noted that survivors of pediatric and young adult cancers seem to have a younger age of onset for cervical cancer than the overall population (33 vs 40 years). It is possible that this effect is explained by a surveillance bias. Survivors of pediatric cancer are likely to receive more frequent screening examinations, but this effect deserves further study (16).
The cervical cancer screening guidelines of MD Anderson do not change for patients with a history of previous cancer. We recommend a liquid-based Papanicolaou test every 3 years for women from ages 21 to 29. Starting at age 30 until age 65, we recommend a liquid-based Papanicolaou test with high-risk HPV testing. If high-risk HPV testing is done and negative, repeat tests should be done every 5 years. If high-risk HPV testing is not done, the screening interval is every 3 years. For patients with abnormal Papanicolaou tests or positive high-risk HPV, appropriate workup according to diagnostic algorithms is recommended.
Ultimately, screening procedures for these and other malignancies will continue to evolve as additional information becomes available. The key point for the provider caring for the cancer survivor is that having a previous malignancy may predispose to additional cancers in the future and certainly is not protective against subsequent primaries. Therefore, attention to cancer screening is of critical importance in preventing or mitigating morbidity and mortality in survivors of a first cancer.
As in adults, the reasons for increased incidence are related to the survivors’ exposure to radiation, chemotherapy, and possible increased genetic predisposition (17). Therefore, screening for certain types of cancers is recommended to begin earlier in survivors of childhood cancer than in the general population, as shown in Table 60-1. As discussed previously in this chapter, screening for breast cancer in women who had chest radiation is especially important because magnitude of risk by age 50 is comparable to carriers of BRCA mutations (18). In addition, children treated with abdominal radiation had increased risk of gastrointestinal cancers (SIR 11.2), warranting earlier screening colonoscopies (19).
Children and adults with a genetic predisposition to a second malignancy, such as those with neurofibromatosis, Li-Fraumeni syndrome, von Hippel–Lindau syndrome, Beckwith-Wiedemann syndrome, FAP, retinoblastoma germline mutations, or multiple endocrine neoplasia syndromes, should be followed in specialty clinics for these disorders or in a childhood cancer survivor clinic. At the MD Anderson Cancer Center (MDACC), a new program led by the Genetics Department is the LEAD (Li-Fraumeni Syndrome Education and Early Detection) program for patients and family members who have germline p53 mutations. Individuals in the program undergo periodic biochemical and imaging surveillance, modeled after the published program that showed significantly improved survival of patient with Li-Fraumeni syndrome who had screening (20).
LATE EFFECTS OF TREATMENT
Cancer and its treatment can have myriad effects on the overall well-being of cancer survivors. Not surprisingly, patients with cancer rate themselves as having poorer health-related quality of life than noncancer survivors, with a significant component of this being physical impairment (21). Broadly, a history of cancer and cancer treatment can have an impact on every system in the body, with common long-term issues that include painful or nonpainful neuropathy, sexual dysfunction, persistent fatigue, lymphedema, surgical disfigurement, and bowel and bladder dysfunction, among other symptoms.
As with the risk of second primaries, the risk of late effects of treatment is particularly important to the survivor of childhood cancers. Over the last 40 years, considerable literature has documented the late effects of cancer treatments in this group. In a report from the Childhood Cancer Survivor Study (CCSS) of 14,359 survivors of childhood cancer with a median follow-up of 24.5 years after diagnosis (range 5 to 39.3 years), cumulative incidence of a severe, disabling, life-threatening, or fatal condition was 53.6% for survivors compared to 19.8% in siblings by age 50 (22). The most common severe chronic conditions were congestive heart failure, second malignant neoplasm, cognitive dysfunction, coronary artery disease, cerebrovascular accident, renal failure, major joint replacement, hearing loss, legal blindness, and ovarian failure (3,23). New onset of these conditions continued to occur at higher incidence than for siblings in every decade for over 35 years from diagnosis. Among survivors who reached age 35 years without a previous grade 3 or 4 condition, 25.9% experienced a subsequent grade 3 to 5 condition (renal failure, stroke, heart attack, congestive heart failure, and pulmonary fibrosis) within 10 years compared with 6.0% of siblings (P < .001) (4). The overall cumulative mortality rate of 20,483 survivors (over 5 years from diagnosis at enrollment) in the CCSS study was 18.1% at 30 years from diagnosis, with the most frequent causes of mortality second malignant neoplasm, cardiac death, and pulmonary death—largely due to treatment-related causes (24).
These late effects threaten both quality and length of life. With over 80% of children with cancer surviving more than 5 years, there is a need to screen for, and if possible prevent, late-occurring problems. With this in mind, the Children’s Oncology Group in 2003 developed evidence-based guidelines that recommend follow-up screening and care of survivors of childhood cancer who are at risk for late effects. These guidelines are risk based depending on the specific exposures to surgery, radiation, and chemotherapy and are designed to begin when a child enters a survivorship clinic at either 5 years from diagnosis or 2 years off therapy when the child is cancer free and without recurrence. They are based on treatment rather than diagnosis and are available to the public (http://www.survivorshipguidelines.org). In our Childhood Cancer Survivor Clinic at MDACC, we follow these guidelines and use them to recommend the standard of care for follow-up in an individualized “Passport for Care.”
CARDIOVASCULAR LATE EFFECTS
Cardiotoxicity is one of the most serious chronic complications of treatment of cancer for both adults and children, but children are particularly vulnerable. Thirty-year survivors of childhood cancer have been shown to have a 15 times higher rate of heart failure, a 10 times higher rate of other cardiovascular diseases, and a 9 times higher risk of stroke than age-matched sibling controls. This risk can persist for 30 or more years after completion of treatment and represents an important source of excess morbidity in patients with cancer (25). The most common causes of cardiotoxicity are anthracycline-based chemotherapy or radiation therapy to the neck and mediastinum.
Anthracyclines are a class of antineoplastic agents that are highly efficacious in the treatment of pediatric and adult hematologic cancers, including acute myeloid leukemia, acute lymphoblastic leukemia (ALL), Hodgkin’s disease, and non-Hodgkin lymphoma, as well as solid tumors, sarcomas, and ovarian cancer. Among children in the United States who are survivors of childhood cancer, approximately 50% have received anthracyclines. Cumulative dose-related cardiac adverse effects may become apparent at the time that the first dose is administered, and clinical data suggest that deterioration of cardiac function is sustained throughout treatment and may become apparent many years after treatment is completed (26).
Known risk factors for anthracycline-induced cardiac adverse effects include high cumulative doses of anthracycline, high anthracycline dose intensity, female sex, age younger than 5 years at diagnosis, radiation therapy, and combining anthracyclines with other cardiotoxic chemotherapy. Patients who were treated for lymphoma, Hodgkin’s lymphoma, sarcomas, or myeloid leukemia generally have the highest risk of cardiotoxicity because of the high doses of anthracyclines they usually receive, often accompanied by radiation. Higher-than-expected occurrences of cardiac adverse effects are observed in patients who receive anthracyclines in combination with new targeted drugs, such as the human epidermal growth factor receptor 2 antibody trastuzumab. These risk factors are helpful in establishing monitoring guidelines, as has been done for children, although they do not predict the risk of cardiac adverse effects for all patients. A new scoring system based on CCSS data provides a score of individual risk for the survivors (27).
Medications such as enalopril and carvedilol can support cardiac function when signs of deterioration in left ventricular function are noted on echocardiograms. While MD Anderson cardiologists prescribe these medications for survivors with decreased ejection fraction and have seen benefit for individuals, the long-term benefit in survivors of childhood cancer has not yet been proven and needs further investigation (28). In adults, these medications have been shown to be effective in preventing chemotherapy-induced left ventricular systolic dysfunction in a randomized trial of adults undergoing aggressive chemotherapy and stem cell transplantation (SCT) (29).
In 2003, the Children’s Oncology Group released risk-based, exposure-related guidelines for children treated with anthracyclines. These guidelines include recommendations for echocardiograms every 1 to 5 years depending on exposure (http://survivorshipguidelines.org). These recommendations differ substantially from those given for patients treated for cancer during adulthood and should be followed. They have been found to be cost effective (30).
Stroke is another late vascular effect of radiation to the head and neck. Survivors of brain tumor and leukemia who received cranial radiation as well as survivors of Hodgkin’s disease who had radiation to the neck have increased risk of cerebral vascular accident when compared to siblings (31). In addition, once a survivor has had a stroke, the risk of having another is significant; therefore, aggressive preventive treatment is recommended (32).