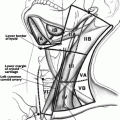
Fig. 25.1
(a) Five ring tracheal resection for Stage IV tracheal invasion in which well differentiated thyroid cancer extended through the entire thickness of the trachea wall. (b) Mobilization of the larynx and trachea to allow-tension free closure
When segmental tracheal resection is performed with end-to-end anastomosis, consideration should be given to raising a pectoralis major flap, especially if there is anticipation for postoperative radiotherapy. The addition of the pectoralis major flap in this situation may reduce complications such as fistula. Oftentimes in these cases, the strap muscles are resected, and the soft tissue coverage over the trachea prevents the skin/subcutaneous tissue from adhering to the trachea with the radiation. The pectoralis major flap also permits great vessel coverage and eliminates dead space.
In certain situations, other techniques may be appropriate. Limited involvement of the anterior wall of the trachea may be addressed by resection of a tracheal window. The resultant defect may be restored by the insertion of a temporary tracheostomy, or for slightly larger defects, a myofascial flap may be rotated in to fill the defect.
If segmental resection results in more than eight tracheal rings being removed, the involved anterior trachea can be resected as a long window and the anterior defect reconstructed with a free flap, although it remains extremely challenging to reconstruct the rigidity of the trachea with free tissue transfer. In some instances, the defect may be a composite anterior tracheal defect in combination with a cricoid and thyroid cartilage defect. A free radial forearm flap can be stitched to a PolyMax mesh (Synthes, Paoli, PA) and Hemashield vascular graft (Boston Scientific, Natick, MA) for rigid support and employed as a rigid, single construct to repair the defect [27]. Circumferential tracheal defects repaired with a free flap supported by prosthetic materials have also been described [28]. Reconstruction may involve the use of a temporary tracheostomy to ensure airway patency in the initial postoperative period.
Pharynx and Esophagus
Isolated pharyngeal involvement is unusual without concomitant laryngeal and esophageal involvement. If isolated pharyngeal involvement occurs, the involved pharynx can be resected via a lateral pharyngotomy. More often however the larynx is also involved and partial laryngectomy with pharyngectomy is performed [15].
Esophageal involvement occurs either as a result of direct posterior extrathyroidal spread or, less commonly, from extranodal spread from central lymph node involvement [29]. When pharyngeal or esophageal involvement occurs, most commonly the muscularis is involved but the mucosa and submucosa are usually spared [15, 30]. If esophageal invasion is limited to the esophageal muscularis, the mucosa and submucosa can be preserved by finding the plane of dissection between the muscularis and submucosa, allowing complete resection and preserving the integrity of the esophageal lumen (Fig. 25.2).
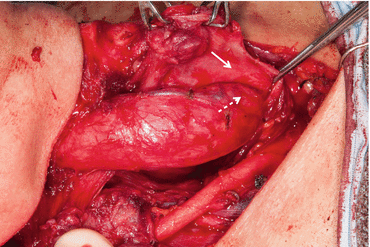

Fig. 25.2
Tumor involving esophageal muscularis, with dissection of the esophageal muscularis (solid arrow) off the esophageal submucosa (dashed arrow) in order to preserve the integrity of the esophageal lumen
If there is full-thickness intraluminal involvement of the esophagus but the area involved is small, full-thickness resection can be performed with tension-free closure in a layered fashion, and consideration may be given to rotating pectoralis muscle to cover the closure. For larger or circumferential defects of the cervical esophagus, reconstruction with a tubed pedicled or free flap is necessary. Free flap options include tubed radial free forearm, anterolateral thigh, or jejunal free flap. If the resection involves the thoracic esophagus, a gastric pull-up will be necessary.
Recurrent Laryngeal Nerve
The recurrent laryngeal nerve can be involved as a result of direct tumor extension from the thyroid or from paratracheal nodal involvement. The recurrent laryngeal nerve is involved relatively frequently in locally advanced thyroid cancer. A study performed at the Mayo Clinic of 262 patients with locally advanced papillary thyroid cancer showed that 123 (46 %) of these patients were found at surgery to have invasion of the recurrent laryngeal nerve with or without invasion of other structures. However, isolated recurrent laryngeal nerve involvement is rare with only 16 of these patients having recurrent laryngeal nerve involvement without invading other local structures [31].
The guiding principle of recurrent laryngeal nerve management is based upon several studies which demonstrate that leaving microscopic disease on the recurrent laryngeal nerve does not lead to decreased survival or increased recurrence, in comparison with resection of the nerve [31, 32].
Nishida et al. performed a retrospective study of 50 patients with differentiated thyroid cancer and intraoperative evidence of recurrent laryngeal nerve invasion. The 50 patients were divided into two groups: 27 patients had the recurrent laryngeal nerve resected with the tumor, and 23 patients had the recurrent laryngeal nerve preserved by dissection of tumor off the nerve. The two groups had similar demographic backgrounds and similar local, regional, and distant involvement. No patients received prophylactic use of radioiodine. The study found that the incidence of postoperative recurrence was not different between the two groups. Rates of local, regional, and distant metastatic recurrences were similar between the groups. Postoperative overall survival of the preserved group was similar to that of the resected group. Mean postoperative survival periods of the resected and preserved groups were 8.55 ± 1.17 and 10.23 ± 1.04 years, respectively [32].
In the retrospective study by Falk et al., 24 patients with papillary carcinoma infiltrating the recurrent laryngeal nerve were analyzed. Five of the subjects had vocal cord paralysis, and 19 had normal vocal cord function. All five patients with paralysis received complete excision of the tumor and involved nerve. Of the 19 patients with normal cord function, 12 received complete and 7 received incomplete excision. Patients with complete excision had resection of all visible tumor and this necessitated excision of the involved recurrent laryngeal nerve. When the two groups of complete versus incomplete excision were compared, no significant difference was found in survival. The authors concluded that because complete excision of papillary carcinoma with resection of the recurrent laryngeal nerve did not improve survival over incomplete microscopic excision, incomplete excision of papillary carcinoma infiltrating a functioning recurrent laryngeal nerve should be performed to preserve the nerve. Postoperative RAI and thyroid-stimulating hormone suppression should be added as adjunct treatment in these situations [31].
Our treatment algorithm for approaching a recurrent laryngeal nerve infiltrated with tumor is often dependent upon the nerve’s baseline function. Therefore, we strongly recommend preoperative evaluation of nerve function by means of either direct or indirect laryngoscopy when concern exists for a locally advanced thyroid cancer. If the nerve is infiltrated or encased with tumor and is functioning preoperatively, every attempt should be made to dissect disease off the nerve, leaving at most microscopic disease behind in order to preserve the function of the nerve. Adjuvant therapy in the form of RAI, T4 suppression, and potentially ERBT should be considered postoperatively and such treatment discussed by the surgeon with the multidisciplinary team. Being most intimately involved with the completeness of resection, the surgeon must play a key role in these decisions.
If the recurrent laryngeal nerve is infiltrated or encased by tumor, and vocal cord paresis or paralysis is present preoperatively (Fig. 25.3), in most cases the nerve should be fully resected in order to completely resect the disease. However, great care should be taken to preserve uninvolved posterior branches and preserve as much unaffected nerve as possible. It is also important to evaluate the status of the contralateral nerve prior to recurrent laryngeal nerve sacrifice, as bilateral involvement requires the surgeon to carefully assess degree of involvement on both sides before deciding on which (if any) unilateral nerve may be potentially sacrificed.
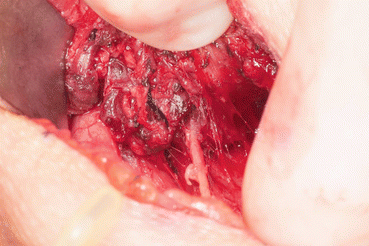

Fig. 25.3
Recurrent laryngeal nerve encased and invaded with well differentiated thyroid carcinoma
In the unusual circumstance that the contralateral vocal cord is paretic/paralyzed despite the absence of tumor, disease should be shaved off the involved recurrent laryngeal nerve in attempts to preserve nerve function. Surgeons should counsel patients about the possibility of the need for a tracheostomy in such cases as shaving tumor off the nerve may result in temporary paresis or permanent paralysis. Because the functional status of the nerve plays such an important role in intraoperative decision-making regarding its handling, preoperative evaluation of the vocal cords when advanced disease is suspected is imperative before embarking on surgery. This information also allows a frank and informative preoperative conversation with the patient about the potential for temporary or permanent airway management.
Reconstruction of Recurrent Laryngeal Nerve
Laryngeal reinnervation after sacrifice of a recurrent laryngeal nerve can allow restoration of muscle tone and achievement of good voice quality. Reconstruction of a recurrent laryngeal nerve can be performed primarily or by means of a nerve graft. If the length of remaining recurrent laryngeal nerve allows direct anastomosis without tension, the nerve can be primarily opposed using three to four stitches of 8–0 or 9–0 nylon or Prolene under an operating microscope or Loupe magnification. If the defect does not allow primary anastomosis without tension, a free nerve graft can be used as a segmental interposition graft. These can be taken from the transverse cervical, supraclavicular, or ansa cervicalis nerves. In situations where the proximal end of the recurrent laryngeal nerve is not available for anastomosis post resection, the proximal end of the ansa cervicalis can also be employed to directly anastomose to the distal end of the recurrent laryngeal nerve (Fig. 25.4a, b) [33].
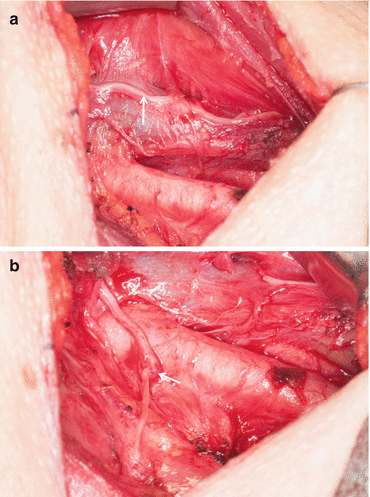

Fig. 25.4
(a) Ansa cervicalis nerve (arrow) in the lateral neck overlying internal jugular vein. (b) Ansa cervicalis nerve to recurrent laryngeal nerve anastomosis (arrow marks the anastomosis)
Several studies have shown good long-term results of both immediate and delayed direct recurrent laryngeal nerve reconstruction. Reinnervation can restore muscle tone, improve glottal closure, confer an improved mucosal wave, and reduce air leak, allowing a better voice outcome to be achieved. Sanuki et al. examined 12 patients with thyroid carcinoma who had preoperative unilateral vocal fold paralysis (n = 6) or who had a unilateral recurrent laryngeal nerve that required sacrifice intraoperatively due to tumor involvement (n = 6). Immediate reconstruction of recurrent laryngeal nerve was performed in all 12 patients by means of direct anastomosis (n = 1), free nerve grafting (n = 9), or ansa cervicalis (n = 2) grafting to recurrent laryngeal nerve. The patients were assessed with the follow-up period ranging from 7 to 103 months (average of 34.6 months). The patients were assessed in the postoperative period in three domains: videostroboscopy findings, aerodynamic findings, and perceptual findings. Scrutinizing patients with known preoperative vocal fold paralysis enabled direct comparison of pre- and postoperative findings in the three domains. For videostroboscopy, although no visible vocal fold movement was detected during the follow-up period, the postoperative mucosal wave and glottal closure score were significantly greater than the preoperative score. Aerodynamic analysis showed that the postoperative recordings of maximum phonation time in this group had significant improvement, and the postoperative mean airflow leak rate had significant reduction. The mean perceptual voice evaluation scores for grade, breathiness, and roughness also showed significant improvement in the postoperative period [33].
Immediate reconstruction of the recurrent laryngeal nerve during thyroid cancer extirpation was described in the study by Yumoto et al. in which 22 patients with advanced thyroid cancer underwent resection of the primary lesion and involved recurrent laryngeal nerve. Recurrent laryngeal nerve paralysis was seen in 12 patients preoperatively and involvement of the RLN was noted intraoperatively in 10. Immediate reconstruction of the RLN was performed in eight patients using the great auricular nerve and by direct anastomosis of the RLN in one; nine patients had no reconstruction. The majority of patients who underwent immediate reconstruction showed minimal or no glottal gap during phonation, whereas those in the nonreconstructed group exhibited a large gap along the entire length of the fold. Phonatory function of harmonics-to-noise ratio, maximum phonation time, and mean airflow rate were also significantly better in the reconstructed group [34].
Wang et al. studied 237 patients with unilateral vocal cord paralysis secondary to thyroid surgery who underwent ansa cervicalis main branch-to-RLN anastomosis. Reconstruction was performed in delayed fashion, with at least 6 months between initial paralysis and reconstruction date. Videostroboscopy, vocal function assessment, and electromyography were performed preoperatively and postoperatively. The mean follow-up period was 5.2 years (ranging from 2 to 12 years). Analysis of videostroboscopic findings indicated that the glottic closure, vocal fold edge, vocal fold position, phase symmetry, and regularity were significantly improved in the postoperative period. The postoperative parameters of vocal function assessment (acoustic analysis, perceptual evaluation, and maximum phonation time) were also significantly improved. Postoperative laryngeal electromyography confirmed successful reinnervation of laryngeal muscle. The authors concluded that delayed laryngeal reinnervation with the ansa cervicalis was effective in restoring laryngeal phonatory function to normal or a nearly normal voice quality in unilateral vocal cord palsy secondary to thyroid surgery [35].
Involvement of Vessels
Invasion of vascular structures in papillary thyroid carcinoma has been shown to have prognostic significance. Both intrathyroidal and extrathyroidal vascular invasion are associated with a higher incidence of distant metastases at diagnosis [36]. Fortunately, well-differentiated thyroid cancer is rarely associated with substantial extrathyroidal vascular invasion or encasement [37].
Venous Involvement
The internal jugular vein is the most common extrathyroidal major vascular structure affected by tumor invasion or occlusion, with or without tumor thrombus. It is most frequently involved by large metastatic lateral neck lymph nodes and less likely to be directly invaded from the thyroid primary [38]. Due to impairment of venous drainage, patients with internal jugular vein involvement may present preoperatively with edema, dilated neck veins, fascial flushing, dyspnea, or dysphagia, though they may be asymptomatic. Further involvement of the superior vena cava with thrombus propagation may manifest with Pemberton’s sign, a triad marked by the presence of facial congestion, cyanosis, and respiratory distress on arm elevation.
CT scanning with intravenous contrast may demonstrate venous involvement with evidence of enlarged veins, tumor invasion, compression, or filling defects [37]. It is important for the clinician not only to appreciate tumor invasion into the internal jugular vein on imaging preoperatively but also to determine if the level of invasion allows sacrifice of the vein to clear disease through a purely transcervical approach. Low-lying level 4 nodal disease with internal jugular vein involvement and extension into the brachiocephalic vein necessitates planning for intrathoracic access. The clavicle or upper ribs may have to be divided or sternotomy performed to safely allow adequate exposure of the vessel and clearance of gross disease.
If only a small patch of vein is involved, the area involved can be excised and the vein repaired either primarily or with a patch using either autologous vein or synthetic material. If however a significant portion of the vessel is involved, the vein should be sacrificed in order to clear disease. In unilateral internal jugular vein involvement, resection of the vessel can be performed with minimal morbidity. However, if both veins are involved, then at least one vein should be reconstructed. Typically, the resections of the veins can be staged, with initial resection and reconstruction of the vein with greater involvement. Staging allows collaterals to form after reconstruction of the first vein so that even if thrombosis develops within the reconstructed vein, sufficient time (usually 6 weeks) is given for collaterals to form before resection of the second vein. Reconstruction can be performed with autologous vein graft or ringed expanded polytetrafluoroethylene graft, though autologous vein graft is preferred as this has a lower risk of thrombosis [39]. Superior vena cava involvement will require cardiothoracic involvement, and successful resection of these tumors through a median sternotomy or right thoracotomy can be performed [40, 41].
Arterial Involvement
Carotid artery involvement is much less common than internal jugular vein involvement and its presence is usually asymptomatic. Carotid artery involvement should be suspected clinically when there is a mass in the area of the carotid artery that is fixed to the bony thoracic inlet. Involvement of the carotid artery is usually picked up on initial imaging (CT with contrast). However, if there is evidence of carotid artery involvement on the initial scan, evaluation may be performed with either CT angiogram or MR angiogram for further delineation. If carotid resection is planned, then MRA or conventional angiography may be employed to determine if shunting is necessary based upon the pattern of collateral intracranial blood flow and the integrity of the circle of Willis [3]. A balloon occlusion test will also be useful in determining if an artery can be sacrificed without shunting.
Depending on the degree of carotid invasion and degree of cross arterial supply, surgical resection can include shave resection, patch excision with autologous vein patch, en bloc excision with ligation, or en bloc resection and reconstruction [37] (Fig. 25.5a, b). Arterial reconstruction is usually performed using polytetrafluoroethylene or vein grafts. It may require a branched “Y” graft if the carotid-subclavian-brachiocephalic junction is involved [42]. Expert colleagues in vascular surgery should be involved in cases that required reconstruction.
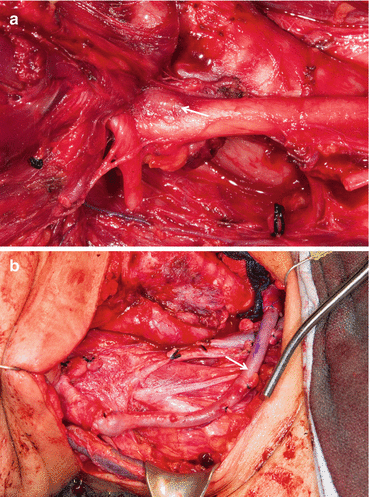

Fig. 25.5
(a) Metastatic thyroid cancer encasing carotid artery and involving the wall of the artery (arrow). (b) En bloc resection and reconstruction of carotid artery with saphenous vein graft (arrow)
Adjuvant Therapy
Role of Radioactive Iodine (RAI)
The current American Thyroid Association (ATA) guidelines recommend the use of RAI in all patients with known distant metastases or gross extrathyroidal extension of the tumor regardless of tumor size. It is also recommended that RAI be considered for selected patients with adverse features such as advancing age, aggressive histologies, or increasing number of large lymph nodes [43]. In patients with invasive WDTC in whom remaining microscopic disease is known or highly suspected after adequate surgical resection, such as with preservation of structures by the utilization of shaving, RAI is an excellent adjuvant treatment. However, RAI is less likely to be effective in achieving complete tumor response in cases in which there is gross residual disease after surgery [3].
Patients with high-risk features who have a known history of poor response to RAI or have a lower chance of responding to RAI, such as those with unfavorable histology, older age, recurrent disease, high FDG uptake, and/or low RAI uptake in known residual disease may be considered for EBRT in specific circumstances (please see section on EBRT below).
Systemic therapy, mainly in the form of kinase inhibitors, has been shown to improve progression-free survival in certain clinical contexts in patients with radioiodine-refractory well-differentiated thyroid cancer. Three randomized placebo-controlled trials with kinase inhibitors have been performed [44–46], two of which specifically addressed advanced local disease in their included cohorts [44, 45]. In a phase 3 trial with sorafenib which included both well and poorly differentiated thyroid cancer, progression-free survival was improved in the drug arm (10.8 months vs. 5.8 months, HR 0.59, 95 % CI 0.45–0.76). However, while 67 of the 417 participants (16 %) were reported to have metastatic lesions sites within the head and neck, only 15 (4 %) had locally advanced disease alone, making interpretation of treatment effect on this variable prohibitive [44]. Similarly in the phase 2 vandetanib trial, in which 61 % of patients had well-differentiated thyroid cancer, only 3 of the 145 participants (2 %) had advanced local disease alone, making conclusions about drug effect in the advanced local setting impossible to generate [45]. Though data specifically related to locally advanced disease is not available, the initial success of these kinase inhibitors in advanced disease makes them important treatment modalities to consider. However, the use of these agents should be performed by clinicians who are well versed in their advantages and adverse effects and always after careful consideration of the more standard therapy of RAI and TSH suppression has been performed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree