Fig. 48.1
Clinical presentation of locally advanced breast cancer
Inflammatory breast carcinoma (IBC) is a rare and aggressive entity characterised by erythema and skin oedema (peau d’orange) occurring over a third or more of the breast (◘ Fig. 48.2). On pathology tumour emboli in dermal lymphatics are usually present, although this is neither necessary nor sufficient (in the absence of clinical symptoms) to diagnose IBC.
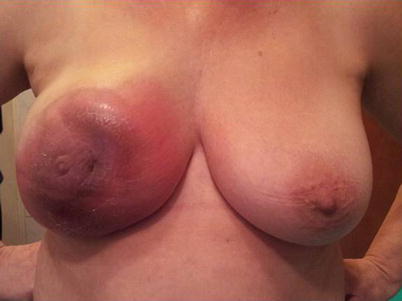

Fig. 48.2
Clinical presentation of inflammatory breast cancer
48.2 Epidemiology, Prognosis and Risk Factors
LABC is relatively rare in developed/high-income countries. In the US National Cancer Database and European CONCORD study, approximately 9.6% and 4% of BC patients, respectively, presented with LABC [2, 3].
This percentage is higher among very young and very old patients and in underserved populations, is lower in populations undergoing regular screening programmes and can be as high as 60% in low-resource countries [4, 5]. Importantly, in the areas and populations of high LABC incidence, such as Africa, Central America and deprived population subgroups in more developed nations, a higher incidence of the most aggressive phenotypes, such as triple-negative BC, is observed [6–12]. In contrast, in areas undergoing rapid development, such as China, the incidence of LABC has reduced substantially (from >20% to ~7%) over the years between 1990 and 2007 [13].
In 2007–2008, 5-year survival rates of US patients presenting with stages IIIA and IIIB were 52% and 48% respectively, with the median survival for stage III disease of 4.9 years [14].
Primary IBC is a relatively rare disorder and accounts for approximately 1–5% of invasive BC [15, 16] and 8.5% of LABC [17, 18]. In the MD Anderson series of 752 stage III BC patients, 24 (3%) had IBC [19]. IBC develops at a younger age compared to other forms of LABC and early BC (mean of 58.8, 66.2 and 61.7 years, respectively) [20]. Several risk factors, often in contrast to «traditional» BC risk factors, have been associated with the development of IBC: race (higher incidence and younger age at diagnosis among Black and Hispanic populations), obesity, young age at first delivery, rural residence and longer cumulative duration of breast-feeding [20, 21].
48.3 Initial (Pretreatment) Patient Evaluation
An accurate determination of the diagnosis, the biologic features of the tumour and the stage of disease is necessary to plan treatment. Mammography and ultrasound are the standard of care and should be performed in all patients, if feasible (◘ Fig. 48.3). Breast magnetic resonance imaging (MRI) is helpful to evaluate disease extent in the breast, in particular the presence of multicentric disease and invasion of the chest wall (◘ Fig. 48.4). As MRI demonstrates the best concordance with pathologic tumour size, it is increasingly used in patients with LABC, in particular those who are deemed to be potential candidates for conservation surgery. As in any other case, the diagnosis of BC should be confirmed by histopathology. Biomarker status (oestrogen receptor [ER], progesterone receptor [PgR], human epidermal growth factor receptor 2 [HER2] and proliferation markers such as Ki-67) of the tumour must be assessed. Core needle biopsy under image guidance is the preferred technique. In patients with suspected IBC or skin involvement, a full thickness skin biopsy is indicated. If BCT is planned, a radiopaque marker should be placed in the tumour before primary systemic therapy (PST) to facilitate surgery (not applicable to IBC) and pathologic assessment in case of complete response. In patients with palpable or suspicious axillary lymph nodes, an ultrasound-guided fine needle aspiration biopsy should be performed.
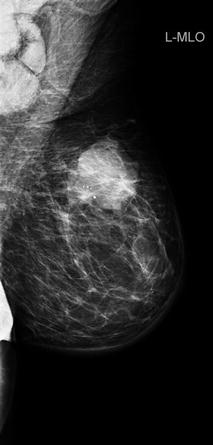
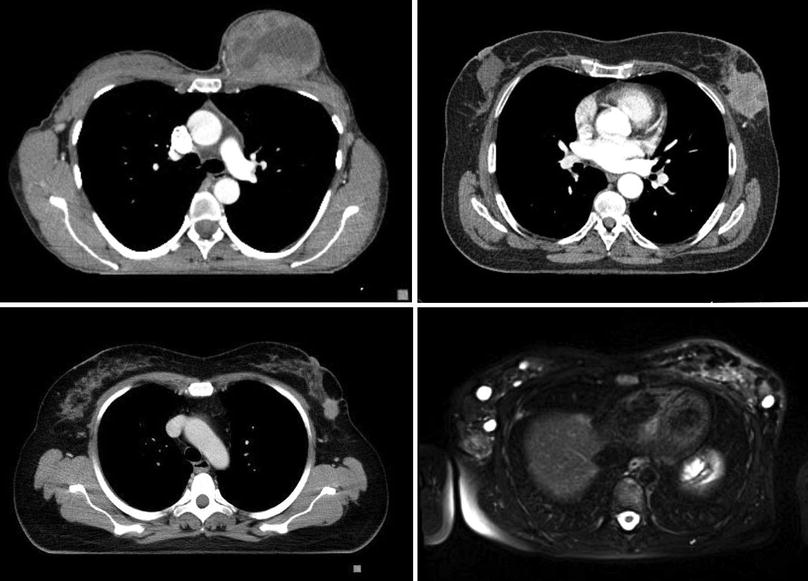

Fig. 48.3
Mammography of inflammatory breast cancer with stromal oedema and skin thickening

Fig. 48.4
CT and MR images of locally advanced breast cancer
A complete history, physical examinations and laboratory tests, including full blood count, liver and renal function tests and serum alkaline phosphatase and calcium, are essential parts of the staging workup. Computed tomography (CT) of the chest, abdomen and pelvis and a bone scan should be performed to rule out metastases. An 18F-fluorodeoxyglucose positron emission tomography scan (FDG-PET) may be used as an alternative and in case of inconclusive results of other imaging studies. Brain imaging is not necessary in asymptomatic patients.
48.4 Pathobiology and Prognostic Factors
The histopathology of LABC, most commonly with infiltrating ductal (NST) and lobular carcinomas, is relatively similar to that of early disease. «Favourable» histologies, such as tubular or medullary carcinomas, rarely present at an advanced stage (apart from neglected cases) and are more often encountered with advanced age [22, 23].
The prognosis in LABC depends on clinical stage and tumour characteristics, in particular histology, grade, expression of hormone receptors (ER, PgR) and HER2 status. Most prognostic and predictive factors are analogous to those of early BC.
The most important prognostic factor for survival is pathological complete response (pCR) after PST. In a recent pooled analysis of 12 large trials of 11,955 patients treated with PST for LABC or «large operable» BC, those who obtained pCR (ypT0/is ypN0) had significant improvements in both event-free survival (EFS) (HR 0.48; 95% CI, 0.43–0.54) and overall survival (OS) (HR 0.36; 95% CI, 0.31–0.42) [24]. The association between pCR and long-term outcomes largely depends on tumour biology and is strongest in triple-negative breast cancer (TNBC) and HER2-positive/HR-negative BC treated with HER2-directed therapy. In hormone receptor-positive or histologically low-grade tumours, pCR is less likely to occur and may not predict long-term outcomes [25].
48.5 Inflammatory Breast Cancer (IBC)
With the absence of definitive molecular or pathological diagnostic criteria for IBC, the diagnosis is usually based on typical clinical features: rapid onset of skin erythema and oedema (peau d’orange) occupying at least one-third of the breast and/or an increased temperature of the breast. Symptom duration is usually short, often measured in weeks. Because clinical diagnosis of IBC represents a broad spectrum of disease presentations, sometimes IBC is misdiagnosed as an infection, mastitis, abscess, ductal ectasia or other malignancies such as breast lymphoma, and biopsy, including full thickness skin biopsy as one of the options, is mandatory in case of any doubt. The hallmark of IBC is dermal lymphatic invasion by tumour cells. This phenomenon is, however, observed in less than 75% of IBC, and it is not required for a diagnosis of IBC which is purely clinical [26].
IBC compared to non-IBC has higher tumour grade, and the frequency of hormone receptor positivity is lower. It is characterised by a high proliferation rate, frequent p53 alteration, E-cadherin overexpression, RhoC overexpression and loss of WISP3 (also known as «lost in IBC», LIBC) [27, 28]. Another feature is the high expression of angiogenic factors reflecting angiogenetic properties, aggressiveness and metastatic potential [29].
The long-term prognosis of IBC remains poor. In the SEER data, a 2-year BC-specific survival (BCSS) in IBC was significantly worse than in noninflammatory LABC (84 versus 91%, HR 1.43), and the 5-year survival rate is approximately 30% [20, 30]. However, due to advances in multidisciplinary management, the 20-year BCSS for IBC patients treated in 1975 and 1995 has increased from 9% to 20% [31]. In a SEER-based study of 7679 IBC patients diagnosed between 1990 and 2010, a significant and stepwise survival improvement in more recent cohorts was seen among women with stage III IBC, regardless of race, age or hormone receptor status [32].
48.6 Treatment Options for Patients with LABC
LABC patients should be managed with multidisciplinary therapy involving systemic and locoregional modalities, and close cooperation between all specialties involved is mandatory. Initial systemic treatment is generally employed as it can increase resectability and breast conservation rates without compromising survival outcomes [33–37]. In IBC, underutilisation of trimodal therapy (systemic, surgery and radiation therapies) negatively impacts survival, and postoperative RT is indicated in all patients, regardless of pathologic findings [38].
◘ Figure 48.5 presents a proposal for LABC management.
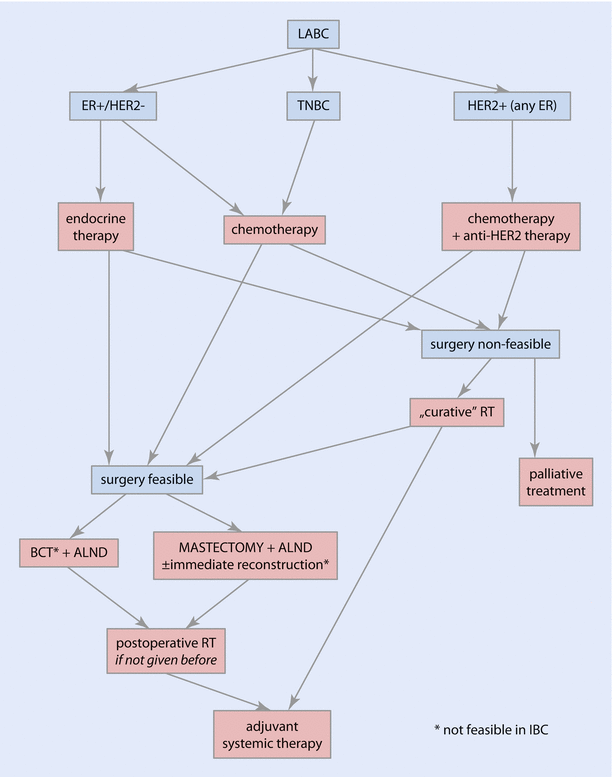

Fig. 48.5
Algorithm of locally advanced breast cancer management. ALND axillary lymph node dissection, BCT breast-conserving therapy, ER oestrogen receptor, HER2 human epidermal growth factor receptor 2, LABC locally advanced breast cancer, RT radiotherapy, TNBC triple-negative breast cancer
48.6.1 Surgery in LABC and IBC
Surgery usually follows initial systemic therapy and precedes radiation treatment. Historically, mastectomy was the routine surgical approach for LABC. However, the efficacy of PST has significantly increased the feasibility of breast-conserving treatment (BCT) [39], making it amenable for up to 50% of LABC patients (however, this is not recommended for IBC even after a complete pathological response) [40].
Indications for breast surgery after PST are similar to those in newly diagnosed BC and are discussed in more detail in ► Chap. 21). The choice of surgical procedure is dependent both on pretreatment disease stage and on its response to neoadjuvant treatment. Whenever breast-conserving surgery is considered, it is essential to mark the primary tumour at the time of pretreatment biopsy to ensure excision of the correct portion of the breast and facilitate pathological assessment for residual tumour [41, 42]. The approach to the axilla following PST is currently evolving; however, routine avoidance of ALND in patients presenting with inoperable LABC is not recommended, regardless of axillary stage before systemic treatment (see ► Chap. 25).
48.6.2 Radiation Therapy in LABC and IBC
There are no available data from phase III radiotherapy trials in exclusively inoperable LABC. In high-risk operable BC, the addition of radiotherapy after surgery results in better locoregional control and an OS benefit [46–48].
Risk factors for locoregional recurrence (LRR) following mastectomy include large primary tumour size, invasion of the pectoralis fascia, higher number of nodal metastases, fewer nodes removed and ER negativity [49–51]. For patients with T4 tumours or multiple positive nodes, treated with PST followed by mastectomy, adjuvant radiation improves locoregional control, relapse-free survival (RFS) and OS [52, 53]. LABC patients who achieve pCR with PST still have a high LRR risk, which can be significantly reduced with radiation therapy (33% versus 3% at 10 years, p-0.006) [54].
Recommendations regarding technical issues in postoperative radiotherapy in LABC patients do not differ from those in early breast cancer patients and are discussed in more detail in ► Chap. 39). LABC patients that cannot be rendered operable by systemic therapy are usually treated with definitive radiotherapy; external beam irradiation is associated with a locoregional failure (LRF) rate of approximately 30%; lower LRF rates of 10–20% can be achieved using external beam radiotherapy in combination with high-dose interstitial implantation [55].
48.6.3 Systemic Preoperative Treatment
The main goal of primary systemic (neoadjuvant) therapy in LABC is tumour downstaging to permit local treatment and to improve surgical outcomes in patients for whom primary surgery is technically not feasible. In selected women, preoperative systemic therapy enables BCT. In addition, preoperative therapy allows for evaluation of its effectiveness and provides additional prognostic information related to pathological response at surgery. Standard systemic therapy options include chemotherapy (ChT), endocrine therapy (ET) and molecularly targeted therapy with HER2-directed agents.
The choice of systemic treatment should depend not only on tumour biology (histology, grade, proliferation markers, ER/PgR and HER2 expression) but also patient-related factors (menopausal status, performance status, comorbidities, preferences) and availability of therapies.
Because reduction in tumour size is the primary goal, in the majority of LABC patients regardless of BC subtype, ChT (with anti-HER2 therapy in HER2-positive patients) is used. However, there are substantial differences regarding options of systemic therapies and their efficacy across BC subtypes. Of note, most data regarding the use of systemic therapies in LABC come from studies with PST, performed in a broader patient population (large operable BC and LABC combined).
The treatment approach in non-metastatic IBC is similar to other LABC classes, with primary systemic ChT (with anti-HER2 therapy in HER2-positive patients) followed by surgery and RT. Optimal treatment regimens in the preoperative setting are not defined, but in general, systemic treatment is the same as for high-risk noninflammatory LABC. Systemic neoadjuvant treatment is discussed in more details in ► Chap. 38).
Triple-Negative LABC
TNBC is highly chemosensitive. In a meta-analysis, achievement of pCR in TNBC patients was associated with risk reductions of approximately 76% for EFS and up to 84% for OS [24]. However, in patients without pCR at surgery, prognosis remains poor [56].
With no currently approved targeted agents for TNBC, ChT remains standard. There is no specific ChT for TNBC, but due to the aggressive nature of TNBC and lack of effective targeted therapies, the most active anthracycline- and taxane-containing regimens are recommended. Multiple studies have demonstrated that primary systemic ChT incorporating both anthracyclines and taxanes is associated with higher pCR and breast conservation rate and improved OS compared with the use of non-taxane-containing regimen [57, 58]. Taxanes can be administered either concurrently or in sequence with anthracycline-based regimens. Based on the results of adjuvant trials, a sequential approach is preferred due to a more favourable toxicity profile and improved efficacy [59]. The optimal sequence of anthracyclines and taxanes is not known. Data from a single neoadjuvant study suggest that the use of taxanes prior to anthracyclines may improve efficacy and is better tolerated [60].
Primary systemic ChT can be administered using standard or dose-dense (ddChT) schedules. In a recent meta-analysis, ddChT increased the probability of achieving pCR by almost 50% [61]. Nonetheless, this has not so far translated into DFS or OS improvements. Given the benefits from ddChT observed in adjuvant trials, it may be appropriate to offer ddChT for high-risk tumour subtypes, such as TNBC [62]. The optimal duration of primary systemic ChT has not been studied, but usually six to eight cycles of ChT are recommended. The whole course of primary systemic ChT should be administered before surgery.
Anthracycline-free ChT such as docetaxel and cyclophosphamide, may be an option in patients with contraindications to anthracyclines [63]. However, recently presented joint analysis of the ABC (Anthracyclines in Early Breast Cancer) trials demonstrated that treatment with taxanes and anthracyclines versus anthracycline-free ChT resulted in superior invasive DFS. The largest benefit from anthracycline-containing regimens was seen in patients with ER-negative disease and/or ≥4 lymph nodes involved. In ER-positive/node-negative patients, the benefit of anthracyclines was questionable. Thus, anthracycline-free ChT should be avoided in high-risk, ER-negative cancer cases [64].
Another combination evaluated in a small single-arm study is carboplatin partnered with a taxane [65]. Preclinical evidence suggests that platinum compounds may be useful, in particular in TNBC- and BRCA-associated BC. Several studies evaluated platinum salts in LABC. Carboplatin incorporated to primary systemic ChT in TNBC improves pCR rates, in particular in BRCA mutation carriers [66, 67]. However, it is associated with increased haematologic toxicity, and data on whether a higher pCR translates into a survival benefit are conflicting. In the GeparSixto study, the effect of carboplatin on DFS was mostly seen in BRCA wild-type patients in spite of higher pCR rates in BRCA mutation carriers, and favourable prognosis in women with pCR was independent of germline BRCA status [68, 69].
Several trials evaluated the role of bevacizumab (monoclonal antibody targeting vascular endothelial growth factor) added to primary systemic ChT. Significantly increased pCR rates were observed across most of the studies, but a group of patients most likely to benefit from bevacizumab was not identified [67, 70]. Given the lack of benefit from bevacizumab in the adjuvant setting, the uncertain benefits from adding bevacizumab to primary systemic ChT with regard to OS and the substantial risk of serious toxicities including early and late postsurgical complications, bevacizumab should not be routinely used. A possible exception may be a situation when better tumour response (due to addition of bevacizumab) could allow for less extensive surgery. Indeed in the CALGB 40603 study, addition of bevacizumab or carboplatin + bevacizumab to standard primary systemic ChT in patients with TNBC increased the rate of conversion from BCT ineligible to BCT eligible by 14% [71].
Currently no data support improved efficacy of routine addition of another non-cross-resistant cytotoxic agent (e.g. capecitabine, gemcitabine) to an anthracycline-taxane-containing regimen [72]. As NAChT offers an opportunity to observe response «in vivo», it has been hypothesised that patients with a poor response after two cycles of ChT might benefit from subsequent administration of a non-cross-resistant ChT regimen, rather than continuation of the same ChT. This concept, representing a treatment tailoring approach, is tempting, but results of clinical trials evaluating response-adjusted sequential therapy are inconclusive [73]. Nonetheless, non-cross-resistant ChT is indicated in patients who have not responded to standard NAChT. Cytotoxic agents typically used after failure of taxanes and anthracyclines include carboplatin, capecitabine and vinorelbine. If response is not observed after subsequent lines of primary systemic ChT, preoperative RT may downstage the primary tumour and allow for resection. As the lack of response after two or three lines of ChT indicates chemoresistance, continuing NAChT beyond this is not recommended, and patients should proceed to local treatment.
Luminal, HER2-Negative LABC
Luminal/HER2-negative tumours, in particular those of lobular histology, have low chemosensitivity and pCR after NAST is rarely achieved (less than 10%). There is a paucity of data regarding the use of ET in LABC, in particular of comparative studies of ET versus ChT. With the scarce data available, the superiority of ChT over ET has not been clearly demonstrated [74, 75]. Importantly, ET use in large operable BC was associated with a higher breast conservation rate compared to ChT [74, 75]. Therefore, in postmenopausal women with luminal/HER2-negative disease, ET may be considered as an alternative to ChT. Aromatase inhibitors (AIs) are the agents of choice, as their use (compared to tamoxifen) results in a higher response rate and higher rate of BCT [76, 77]. The efficacy of particular AIs (anastrozole, letrozole and exemestane) in the primary systemic therapy setting is comparable [78]. Very few data exist on primary systemic ET in premenopausal patients, and this approach is not recommended outside clinical trials.
Time to response in ET is longer than in patients undergoing primary systemic ChT. Therefore, ET should be continued for 6–8 months before local treatment, provided that response is observed and patients are carefully monitored. The duration of ET prior to surgery may be individualised based on clinical response and the patients’ clinical status. Longer ET may increase the rate of BCT.
HER2-Positive LABC
Rate of pCR to PST in HER2-positive BC is high, in particular in hormone receptor-negative patients. Combining primary systemic ChT with HER2-directed therapy not only increases pCR but also impacts long-term outcomes. Randomised trials have demonstrated that the addition of trastuzumab (monoclonal antibody targeting HER2) to primary systemic ChT resulted in pCR and EFS improvement [24, 79, 80]. A trend towards OS benefit was also observed in a pooled meta-analysis of two studies [81].
Trastuzumab is usually combined with standard anthracycline-taxane-based primary systemic ChT. In patients with contraindications to anthracyclines, trastuzumab can be partnered with taxanes and either carboplatin or cyclophosphamide for six cycles [82]. Concurrent administration of trastuzumab and anthracyclines is not recommended due to the risk of cardiotoxicity and no clear benefit compared to sequential use [83].
Combination of ChT and lapatinib (tyrosine kinase inhibitor of HER2 and epidermal growth factor receptor) is inferior to ChT-trastuzumab and is not recommended [84–86]. Double anti-HER2 blockade with trastuzumab and lapatinib in the majority of trials significantly increased pCR [84–86]. However, this had no effect on DFS or OS, and lapatinib was associated with more adverse events, primarily gastrointestinal toxicity. Thus, lapatinib-containing regimens remain investigational and should not be used in routine clinical practice. Another option is the dual anti-HER2 antibody inhibition with trastuzumab and pertuzumab (monoclonal antibody that blocks the formation of HER2-HER3 heterodimers). In the phase II Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation (NeoSPHERE), addition of pertuzumab to trastuzumab and docetaxel resulted in increasing the pCR rate to 46% compared to 29% in docetaxel-trastuzumab arm. Interestingly, combination of pertuzumab and trastuzumab alone without a ChT backbone resulted in a pCR rate of 16.8% in the breast [87, 88]. In the Trastuzumab plus Pertuzumab in Neoadjuvant HER2-Positive Breast Cancer (TRYPHAENA) trial, even higher pCR rates of 65–84% in the ER-negative group and 46–50% in the ER-positive group, depending on treatment sequence, were achieved [89]. Based on these results, pertuzumab was approved in the USA and EU in the primary systemic therapy setting in combination with trastuzumab and docetaxel or docetaxel and carboplatin. Despite the promising pCR rates, mature long-term outcome data and confirmatory results from phase III studies in either the primary systemic or adjuvant setting are needed before primary systemic pertuzumab-containing regimens can be considered standard. In recently published APHINITY trial the absolute benefit from adding pertuzumab to adjuvant ChT with trastuzumab was modest and clinically not meaningful, with 3-year invasive DFS difference of only 1% [90].
48.7 Response Assessment
Clinical response should be assessed regularly during preoperative therapy by physical examination of the breast and axilla to avoid missing disease progression and after completion of the whole course of neoadjuvant therapy to choose the optimal locoregional treatment. For patients receiving primary systemic ChT, clinical evaluation should be performed at every 2–4 ChT cycles, in women undergoing ET, every 2–4 months, using standard RECIST 1.1 criteria [91]. If progression is suspected and before the decision on locoregional therapy, imaging is mandatory. Breast MRI if performed prior to neoadjuvant treatment and after completion of therapy allows for best evaluation of response and assessment of the extent of residual disease when breast-conserving surgery is planned.
There is modest concordance between imaging with ultrasound, mammography or MRI and pathologic assessment of response [92, 93]. The highest specificity among imaging techniques is attributed to contrast-enhanced MRI and FDG-PET. In a meta-analysis of 25 studies in 1212 patients receiving PST, contrast-enhanced MRI had a specificity of 91%, but a relatively low sensitivity of only 63% to predict pCR. The value of FDG-PET in response prediction with a sensitivity and specificity of about 80%, though very promising, is not sufficient to justify its routine use [94–96]. Additionally, due to the heterogeneity in breast cancer biology, FDG-PET seems to predict pCR with higher accuracy in certain tumour subtypes such as in HER2-positive and TNBC, whereas in luminal HER2-negative tumours, in which pCR is less likely and has little prognostic value, the ability of FDG-PET to assess response and detect pCR is not sufficient. Serial biopsies of the tumour with the assessment of tumour markers such as Ki67, though may provide information on the biological effect of therapy, are not recommended outside clinical trials.
Post-treatment pathologic staging in patients treated with PST should be assessed using The American Joint Committee on Cancer and the International Union for Cancer Control (AJCC-UICC) tumour, node, metastasis (TNM) staging criteria. This system uses «y» to designate the pathologic stage after preoperative therapy (ypTNM). There are multiple definitions of pathological complete response (pCR), varying from the absence of residual invasive disease only in the breast regardless of pathologic findings in the axillary nodes (ypT0/is ypN0/+) to full eradication of invasive tumour or invasive and in situ cancer in the breast and axillary lymph nodes (ypT0 ypN0), but only the latter or ypTis ypN0 should be considered as pCR in clinical practice, as it is most closely associated with improved outcomes [24].
In the majority of patients undergoing PST for LABC, pCR is not achieved. When invasive cancer is present, the pathology report should include a description of the effect of PST in the tissue of the breast and lymph nodes. There are several tools to quantitate pathological response which seems to correlate with BC outcome: the residual cancer burden (RCB) score, the Breast Cancer Index (BCI) and the preoperative endocrine prognostic index (PEPI) score (for patients treated with primary systemic ET) [97–100]. However, none of the methods predicting patients’ prognosis based on pathologic findings have been sufficiently standardised; thus, their use in clinical practice is limited.
48.8 Adjuvant Therapy
With the lack of data supporting the use of additional ChT, even in patients with gross residual disease, adjuvant ChT after primary systemic ChT is not routinely recommended [101, 102]. However, in a study (CREATE X) performed in Japan and Korea in patients with residual disease after primary systemic ChT postoperative treatment with capecitabine resulted in increased DFS and OS [103]. As accumulating evidence suggests benefit from capecytabine added to ChT in adjuvant and neoadjuvant setting in patients with triple negative BC [103, 104], single agent capecytabine can be considered as adjuvant therapy in case of no pCR after preoperative ChT in this population. Because of the poor prognosis in patients with residual disease after PST, current research focuses on the development of novel strategies for these patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







