Localized Prostate Cancers
EPIDEMIOLOGY
In the United States, an estimated 238,590 men will be diagnosed with prostate cancer in 2013 with 29,720 deaths attributable to prostate cancer (1). These statistics highlight a paradox of prostate cancer. Although it is the second leading cause of cancer death for men in the United States, only a relatively small percentage of men diagnosed with prostate cancer will die of their disease. This chapter will present guidelines for the management of localized prostate cancer. It will describe the controversies associated with prostate-specific antigen (PSA) screening, describe the work up and staging of prostate cancer, and finally discuss treatment options for localized disease.
PROSTATE-SPECIFIC ANTIGEN (PSA)
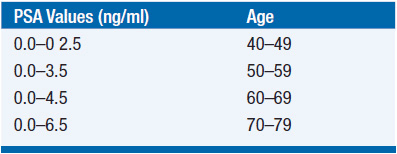
PSA is an abundant exocrine protein of the prostate, which has function in seminal clot lysis. Serum PSA measurement is a useful, although highly controversial, biomarker commonly used as a screening tool for prostate cancer. In addition to prostate cancer, PSA elevations may be the result of a variety of nonmalignant conditions including benign prostatic hypertrophy, inflammation, or urinary tract infection. Traditionally, 4.0 ng/ml has been considered the upper limit of normal for serum PSA; however, recent data demonstrate that many men with a serum PSA in the normal range have prostate cancer if biopsied (2). Conversely, many men with elevated PSA levels do not. As PSA normally rises with age, an age-specific algorithm may be a more effective screening approach. Age-specific PSA normal ranges have been shown to aid in finding important early cancer in younger men and avoiding unnecessary procedures and over-diagnosis in older men (Table 40-1).
CURRENT RECOMMENDATIONS FOR SCREENING
Prostate cancer screening is a controversial topic with several large recent studies informing the debate over the benefits of screening (3–5). Although PSA screening has led to the detection of earlier stage prostate cancer, it is not clear if this has led to better outcomes for screened men. Many screen-detected cancers may be incidental cancers that would have never resulted in clinical sequelae within the man’s lifetime. Given this lack of clear evidence, the United States Preventative Service Task Force has recently recommended against routine PSA screening, concluding that “many men are harmed as a result of prostate cancer screening and few, if any, benefit” (6). This recommendation has met significant resistance from the urologic and oncologic communities (7, 8). These groups point out the difficulty of interpreting PSA screening studies, given high levels of contamination, and that this broad recommendation may not apply to young healthy men. As such, The American Cancer Society and the National Comprehensive Cancer Network (NCCN) currently recommend a careful discussion between the patient and his physician before proceeding with screening. The American Urological Association (AUA) has released a similar guidelines calling for shared decision making about PSA screening between providers and patients age 55–69 (available: http://www.auanet.org/education/guidelines/prostate-cancer-detection.cfm). For men who elect screening, PSA and digital rectal examination (DRE) should be offered every 1–2 years. Continued screening is most appropriate in men with a life expectancy of at least 10 years. Factors that increase the risk of prostate cancer include sub-Saharan African descent or history of prostate cancer in a first-degree relative before the age of 65 years. In men with these risk factors, screening should begin at age 40 years.
 DIGITAL RECTAL EXAMINATION (DRE)
DIGITAL RECTAL EXAMINATION (DRE)
Although the majority of prostate cancer is detected by PSA screening, others are diagnosed based on an abnormal DRE even in the setting of a normal PSA. Both PSA and DRE should be incorporated into the screening process. A palpable nodule or a discrete indurated area constitutes a suspicious DRE that should prompt further evaluation.
DIAGNOSIS
Prostate cancer is diagnosed exclusively by the use of a transrectal ultrasound guided needle biopsy (Figure 40-1). This is an outpatient office-based procedure performed under local anesthetic. It has a very low-complication rate estimated at 1 serious complication per 1000 procedures. There is no radiographic modality adequate for diagnosis without the use of biopsy. Ultrasound may show a hypoechoic area that corresponds to localized carcinoma, but this is an unreliable finding. The proper technique for prostate biopsy has been well studied. The standard approach is a systematic sampling of all areas of the prostate in a grid pattern (Figure 40-2). Most urologists employ a technique of sampling 6–12 regions of the prostate. The cancer detection rate is substantially higher with a 10–12 core biopsy technique.
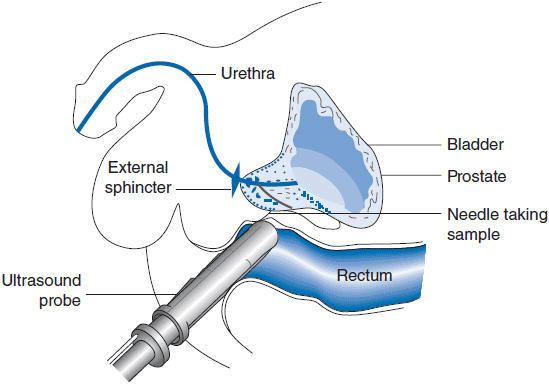
FIGURE 40-1 Transrectal ultrasound guided biopsy.
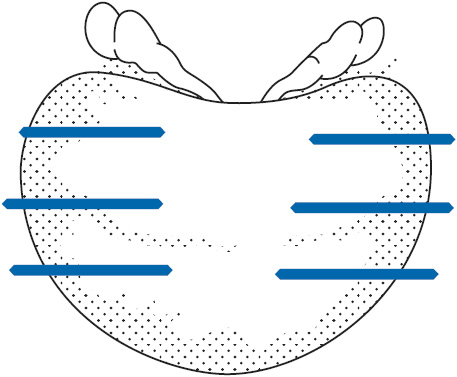
FIGURE 40-2 Sextant biopsy pattern.
 GLEASON SCORE
GLEASON SCORE
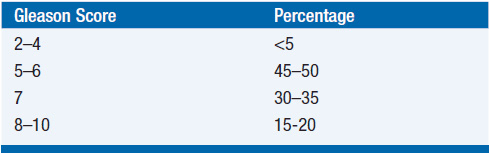
The histologic grading of prostate cancer is described using the Gleason scoring system (Table 40-2) (9, 10). This system assigns two numeric scores to the carcinoma. As there is often heterogeneity within the cancer, the first score describes the dominant pattern and the second the secondary pattern. For example, Gleason 3 + 4 indicates the primary pattern is 3. Gleason 4 + 3 indicates that the higher grade predominates. This is clinically important because prognosis is substantially related to the primary Gleason score. In discussing prostate cancer staging, the Gleason sum, that is, the sum of the individual scores, is commonly referenced. Although the Gleason sum is one of the most important prognostic features, the primary and secondary scores should also be reported as they may refine a patient’s prognosis. For instance, Gleason sum of 3 + 4 and 4 + 3 are both 7, although the latter cancer has a worse prognosis.
In the absence of prostate cancer, there are other common histologic findings that warrant consideration. Atypical small acinar proliferation (ASAP) frequently represents an inadequately sampled carcinoma escaping definitive diagnosis. A repeat biopsy is indicated. High-grade prostatic intraepithelial neoplasia (HGPIN) is commonly found on prostate biopsies. The significance of this finding is controversial. Standard practice calls for continued close monitoring and rebiopsy of these patients. Perineural invasion (PNI) is a finding often reported in men who have prostate cancer. Some investigators believe this is associated with a higher risk of extra-prostatic tumor spread, but this has been an inconsistent finding.
STAGING
The most important independent variables used in staging prostate cancer are the patient’s PSA, the DRE findings, and the Gleason score. Using these clinical features, significant prognostic judgment can be made. The Partin tables assess the likelihood of pathologic stage based on these clinical variables. They are very useful in counseling patients about appropriate treatment options (available at: http://urology.jhu.edu/prostate/partintables.php). Metastatic workup includes a CT scan of the abdomen and pelvis to assess for pelvic and retroperitoneal lymphadenopathy. A bone scan may pick up bone metastases. Because of the extremely small likelihood of positive findings, these studies are rarely useful in early stage disease. In general, these studies are indicated only in patients with either Gleason 4 + 3 or higher cancer, PSA greater than 10, or clinical high local stage tumor (T2b or higher).
Endorectal MRI has been extensively studied for its role in local staging of prostate cancer. While its sensitivity for detecting subtle extra-capsular extension is higher than that of ultrasound, the clinical implications of such findings remain unclear.
 MANAGEMENT OPTIONS
MANAGEMENT OPTIONS
Definitive local treatment is primarily a curative strategy in patients with localized disease, but it is occasionally employed to prevent symptoms from locally advanced disease even in men with evidence of distant metastasis.
 WATCHFUL WAITING/ACTIVE SURVEILLANCE
WATCHFUL WAITING/ACTIVE SURVEILLANCE
Prostate cancer frequently behaves in an indolent fashion. Watchful waiting or active surveillance is an attractive strategy for men with less than a 10-year life expectancy or with low-volume Gleason 3 + 3 disease. Population-based studies suggest only a tiny minority of men with Gleason 3 + 3 carcinoma will develop metastatic or life-threatening disease within 10 years if left untreated (11). Despite this, a randomized trial performed in Europe demonstrated that radical prostatectomy reduced the risk of death, metastatic dissemination, and local progression as opposed to watchful waiting in men with early prostate cancer (12). As the majority of these men did not have screen-detected cancers, this study may not fully represent the population with prostate cancer in the United States. A more recent study of patients with PSA screening-detected cancers showed no benefit to prostatectomy except for men with high Gleason grade or PSA greater than 10 ng/ml (13). Active surveillance, a different concept than watchful waiting, relies on serial PSA follow-up and repeat biopsies allowing significant cancers to declare themselves early in follow-up. Patients with significant cancers are offered treatment in a timely fashion, while patients with indolent prostate cancer are spared radical treatment and the associated sequelae. Prostate cancer specific survival of 97% at 10 years have been reported and up to 70% of men are able to avoid treatment (14). Multidisciplinary care has been shown to increase rates of active surveillance usage in low-risk patients (15).
 RADICAL PROSTATECTOMY
RADICAL PROSTATECTOMY
Radical prostatectomy is the standard surgical procedure comprising complete removal of the prostate and seminal vesicles. This procedure is done using several different approaches. Radical retropubic prostatectomy involves a midline incision from the umbilicus to symphysis pubis. Radical perineal prostatectomy is performed through a midline incision between the scrotum and the anus. Minimally invasive surgical approaches are now in wide use. A laparoscopic approach through the lower abdomen can be accomplished by standard laparoscopic technique or with the aid of a surgical robot. In skilled hands, the mortality and major morbidity rates with any of these procedures are extremely low. Major potential complications of surgical removal of the prostate are urinary incontinence, erectile dysfunction, significant hemorrhage, and bladder neck contracture. In properly selected patients, severe incontinence occurs in less than 1% of cases. Mild stress urinary incontinence in which the patient may require pads in his underwear to catch some occasional urinary leakage is found in less than 10% of patients who are treated at high-volume centers. Erectile dysfunction rates vary widely with patients’ preoperative sexual function, the choice of surgical approach (nerve sparing or non-nerve sparing), and the patient’s age. In early stage disease, bilateral nerve sparing surgery may result in preservation of erectile function in up to 80% of younger men with normal preoperative sexual function. More aggressive surgical resection that includes one of the paired neurovascular bundles, which run posterolaterally to the prostate, results in less than 50% of patients recovering spontaneous erectile function. If both nerve bundles are sacrificed, spontaneous erectile function is unlikely to recover. Bladder neck contracture results when dense scar tissue obstructs the point of anastomosis of the urethra and bladder. This may result in acute urinary retention in the early postoperative period. Repeated urologic procedures may be necessary to restore adequate urinary function. Significant hemorrhage may be encountered during radical prostatectomy. It has been common practice for surgeons to bank autologous blood prior to radical prostatectomy. Blood loss on average is much reduced with perineal and robotic approaches to radical prostatectomy. Regional pelvic lymph nodes are routinely sampled during radical retropubic and laparoscopic radical prostatectomy. It is not possible to sample pelvic lymph nodes through the perineal approach.
 RADIATION THERAPY OPTIONS
RADIATION THERAPY OPTIONS
External Beam Radiation Therapy
External beam radiation therapy has a well-established track record in the management of localized and locally advanced prostate cancer (16–18). It is a noninvasive form of treatment delivered in daily fractions over the course of several weeks. Modern trials have demonstrated that higher doses, on the order of 79 Gy, are more efficacious for cancer control when external radiation is used as monotherapy and can be delivered without a decrement in patient-reported quality of life (19–21). Conformal radiation therapy utilizing CT or MRI planning is necessary for the safe delivery of sufficient radiation dose in this setting. Daily prostate imaging (i.e., image-guided therapy) using transabdominal ultrasound, radiographic visualization of implanted fiducial markers, electromagnetic transponders or cone-beam CT allows more accurate targeting of the prostate. This facilitates the delivery of high radiation doses to the prostate while minimizing dose to nontarget tissue, such as the bladder and rectum, that is the primary cause of late morbidity. A variety of external beam radiation techniques are acceptable forms of treatment including multifield 3D conformal radiation, intensity modulated radiation therapy (IMRT), and proton beam radiation therapy. Currently such treatment is typically delivered over the course of 8–9 weeks; however, several trials studying the efficacy of shorter (i.e., hypofractionated) courses are ongoing. Potential toxicity of external beam radiation therapy include acute changes to bowel habits (e.g., looser stools) and increased urinary frequency/urgency as well as potential radiation proctitis, cystitis, and urethritis, though the rates of significant late complications are less than 10%. Similar to surgery, erectile dysfunction is a common side effect of treatment, although it typically manifests later. Rates of erectile dysfunction vary with patient age and the presence of medical comorbidities, particularly cardiovascular disease, obesity, diabetes, and smoking.
Prostate Brachytherapy
Prostate brachytherapy is another common treatment modality for early prostate cancer. As monotherapy, prostate brachytherapy may be less effective in patients with Gleason grade 7 or higher, palpable cancer, or PSA greater than 10 (22). For well-selected patients, brachytherapy may be more attractive than a protracted course of external beam radiation. Modern prostate brachytherapy is typically delivered under transrectal ultrasound guidance using a transperineal approach (Figure 40-3). As modern series have matured, results have been comparable to surgical and external beam radiation for early stage disease. Commonly, this procedure is performed in an operating room under either general or spinal anesthesia. An ultrasound probe is placed in the rectum, and needles containing radioactive seeds are guided into the prostate using a transperineal template or grid. These seeds typically contain either iodine-125 or palladium-103, whose half-lives are 60 and 17 days, respectively. They remain in the prostate permanently and deliver the full dose of radiation over several months. This procedure is termed transperineal permanent low-dose rate (LDR) prostate brachytherapy. It differs from high-dose rate (HDR) brachytherapy where temporary catheters are placed in the prostate using a similar placement technique. The catheters serve as avenues for an HDR radioactive wire, which contains a high-activity source, typically iridium-192. High-dose rate brachytherapy requires the delivery of several fractions, necessitating repeated catheter placement. This treatment is most frequently combined with external radiation but is also used as monotherapy.
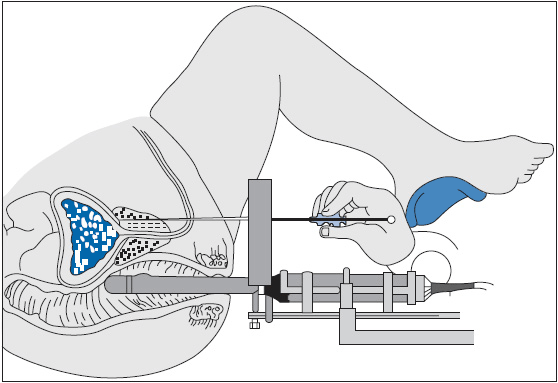
FIGURE 40-3 Ultrasound guided transperineal permanent prostate implant technique.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree