1. COMPLETE RESECTION OF AN INTACT SPECIMEN WITH NEGATIVE BOWEL WALL AND DEEP MARGINS
Rationale
For decades, local excision of small rectal tumors has been performed by using a transanal endoluminal approach, primarily by utilizing standard surgical lighting, instruments, and retractors. For well-selected lesions close to the anal verge, especially in thinner patients, this is readily accomplished and remains an efficient, cost-effective technique for local resection.
33 However, poor exposure of more proximal lesions, especially in patients with an unfavorable body habitus, may result in suboptimal excision.
34 The resulting specimens are more likely to be fragmented, and the surgical margins are more likely to be inadequate.
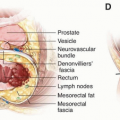
Irrespective of the specific approach (transanal excision [TAE], transanal endoscopic microsurgery [TEM], or transanal minimally invasive surgery [TAMIS]) chosen for local excision of rectal cancer, a complete excision of the tumor with negative bowel wall and deep margins should be performed. To ensure complete resection, the excision should aim to achieve a 10 mm margin along the mucosal and the fullthickness excision should be performed to the level of the peri-rectal fat. The specimen should be removed intact to enable histopathologic examination and determination of the true margin status. Excision that is performed piecemeal cannot be assured to be complete, and the margins cannot be adequately assessed.
Historically, the Kraske transcoccygeal or York-Mason transsphincteric approach was applied to improve exposure to overcome the limited upward reach provided by the conventional TAE technique. Newer techniques including TEM and, more recently, TAMIS, have overcome the shortcomings of standard TAE without the added morbidity associated with the transsphincteric or transcoccygeal approaches. These approaches have made TAE more precise with less fragmentation of the surgical specimen and have decreased the rate of local recurrence relative to standard TAE.
85,
133,
134,
135,
136,
137,
138,
139,
140TEM was pioneered by Gerhard Buess in the mid-1980s. TEM utilizes a closed system that insufflates carbon dioxide into the rectum, enabling rectal distension and exposure (the “pneumorectum”). A fiberoptic high-definition scope with a wide, magnified field of view is inserted through a rectoscope with a 4-cm diameter. The rectoscope is regulated by an endosurgical unit that provides insufflation, irrigation, suction, and pressure monitoring. Long-shafted instruments, inserted through the working ports, are capable of grasping, dissecting, electrocauterizing, and suturing tissues.
TAMIS utilizes a variety of technologies initially developed for laparoscopic surgery (e.g., fiberoptic laparoscopes, multichannel single-port platforms) that similarly enable creation of a pneumorectum, provide high-quality images, and utilize ordinary long-shafted laparoscopic instruments to allow for excision of the lesion, suction and/or irrigation, and control of bleeding points.
141,
142,
143 A number of variations have been utilized in attempts to enhance the safety and capability of TAMIS and to reduce costs.
In general, TEM and TAMIS have complication rates similar to those associated with traditional TAE. However, potential disadvantages associated with the newer platforms include the need for specialized training to learn the techniques and increased cost. The complication rates and functional results of TAE are difficult to compare with those of the newer platforms because the data are older and reflect a time when there was less emphasis on and sophistication in the measurement of outcomes.
TEM and TAMIS appear to have a number of clear oncologic advantages over TAE.
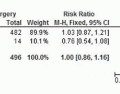
144 These include a substantially lower rate of positive margins, a diminished risk of tumor fragmentation, and a decrease in local recurrence rates. The incidence of positive or indeterminate margins with TAE has been two to eight times greater than with TEM.
145Because the platforms, techniques, and instrumentation continue to evolve in the burgeoning field of transanal endoscopic surgery, a meaningful comparison of TEM with TAMIS and all of its iterations is of limited utility.
146,
147,
148 Nonetheless, it appears
that one advantage of TAMIS is the ability to use or adapt readily available laparoscopic equipment and instrumentation, whereas TEM requires specialized equipment. This difference could mean a cost savings for TAMIS and shorten the learning curve relative to TEM, because the equipment is already available and familiar to the surgeon.
Complication rates with TEM range from 0% to 29%
134,
149,
150,
151,
152,
153,
154,
155,
156,
157 and are usually minor and self-limited (urinary retention/infection, bleeding, and suture line dehiscence).
158,
159,
160,
161,
162,
163,
164,
165,
166,
167,
168,
169Operative efficiency with TEM continues to improve up to 16 cases,
170 but the overall learning curve appears to be quite steep, with improved outcomes reported after performance of 35 cases.
171,
172 The conversion rate from TEM to an abdominal approach in one large series (
n = 693) was 4.3%.
172 The best available evidence strongly suggests that TEM has minimal or no long-term impact on continence or functional outcomes (including urologic and sexual dysfunction) as measured by clinical, manometric, and validated quality-of-life metrics.
161,
173,
174,
175,
176,
177 The addition of radiation therapy to TEM (or other forms of TAE) has a substantial impact on the complication rate and functional results.
178,
179A pooled analysis of 33 TAMIS-related reports included a 4.3% rate of positive margins and a 4.1% incidence of tumor fragmentation.
180 Concern has been expressed about the ability to close full-thickness defects in more proximal tumors with peritoneal entry by using the TAMIS platform.
181 The overall complication rate with TAMIS was 7.4%, with minor, self-limited bleeding and urinary retention representing more than half of the reported complications. A learning curve of 20 cases has been suggested, generally shorter than that reported with TEM. The data on the functional outcomes with TAMIS are much more limited than with TEM; however, there does not appear to any significant adverse impact on quality of life or continence scores.
182,
183,
184