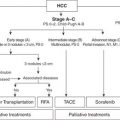
FIGURE 10.1 Intrahepatic portal vein anatomy as depicted by Rex in 1888.
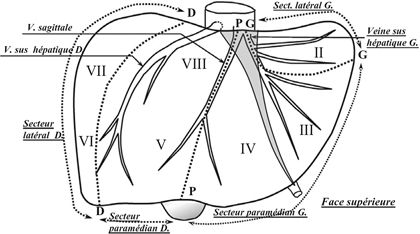
FIGURE 10.2 Liver segments numbered clockwise from I to VIII and sectors (dotted lines) along the planes of the three hepatic veins as described by Couinaud. (Adapted from Couinaud C. Lobes et segments hepatiques: notes sur l’architecture anatomiques et chirurgicale du foie. Presse Med 1954;6(2):709–712.)
The International Hepatopancreatobiliary Association (IHPBA) consensus Brisbane (2000) classification for liver surgery terminology is now widely adopted, with the liver divided into sections rather than sectors. The main difference is that segments II and III now belong together as the left lateral section with segment IV remaining as the medial segment without segment III (Fig. 10.3). In an effort to standardize terminology among surgeons and surgical literature, the terms right/left lobectomy, bilobar, and trisegmentectomy are now mostly replaced with the terms right/left hepatectomy, bilateral, trisectionectomy, sectionectomy, and segmentectomy. Detailed knowledge of the segmental and vascular anatomy is critical to preoperatively planning the amount of liver parenchyma that will need to be resected and how much FLR will remain after resection. Precise terminology is critical for communication among surgeons, radiologists, and the multidisciplinary team.
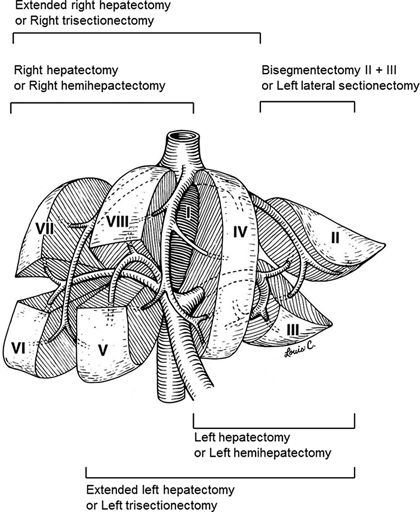
FIGURE 10.3 Resection planes using the International Hepatopancreatobiliary Association (IHPBA) Consensus Brisbane (2000) classification for liver surgery. (Adapted from Abdalla EK, Denys A, et al. Total and segmental liver volume variations: implications for liver surgery. Surgery 2004;135(4):404–410.)
PHYSIOLOGY
For safe resection, the liver parenchyma must have sufficient size, adequate synthetic function, unimpaired biliary drainage and perfusion, and sufficient regenerative capacity. A global assessment of parenchymal quality is important to determine the functional ability of the liver to tolerate resection and to guide the surgeon in choosing a safe maximum volume that can be resected. This can involve the patient’s history, physical exam, biochemical tests, and radiologic imaging.
For patients with cirrhosis, the Child-Pugh score classifies the severity of chronic liver disease. This score incorporates albumin, ascites, bilirubin encephalopathy, and international normalized ratio (INR) and, by using a scoring system, stratifies three classes of mortality risk (Table 10.1). Usually, only Child-Pugh A patients are considered operable because the pathophysiologic consequences of cirrhosis are minimal, and thus they are described as “compensated.” Some surgeons will operate on Child-Pugh B patients but with a known increased perioperative risk. The operative mortality of Child-Pugh C patients can approach 50% even for “minor” general surgery operations such as umbilical hernia repair and cholecystectomy. Child-Pugh C patients cannot tolerate any liver resection. The best treatment for hepatocellular carcinoma (HCC) in patients with Child-Pugh B and C cirrhosis is liver transplantation if they qualify.
TABLE 10.1 Child-Pugh score for cirrhosis
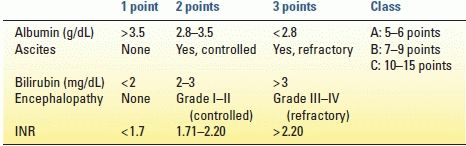
INR, international normalized ratio.
From Child CG, The liver and portal hypertension. Philadelphia, PA: Saunders, 1964.
An alternative system for grading cirrhosis is the Model for End-Stage Liver Disease (MELD), which consists of bilirubin, creatinine, INR, and liver disease etiology, in a formula originally created to predict 3-month mortality after a transjugular intrahepatic portosystemic shunt (TIPS) procedure and validated for predicting mortality in hospitalized and ambulatory patients awaiting liver transplant. As an alternative to Child-Pugh score, MELD score can be used to predict perioperative and long-term mortality after resection of HCC. While patients with high MELD scores (≥ 9) are inoperable for surgical procedures other than transplant, those with MELD scores less than 9 can be considered for hepatectomy.
While these rules of thumb are easy to follow if a diagnosis of cirrhosis already exists, the real clinical problem occurs when chronic liver disease is unrecognized until surgical exploration. Thus, patients should be carefully screened preoperatively for cirrhosis and portal hypertension based on history (jaundice, hepatitis, upper gastrointestinal [GI] bleeding, encephalopathy), physical examination (skin varices or superficial venous collaterals, ascites, mental examination), serum laboratory tests (bilirubinemia, thrombocytopenia, anemia from bleeding), and imaging (ascites, splenomegaly, scalloping of the liver, left lateral section and caudate hypertrophy, intra-abdominal varices, and venous collaterals). In general, patients with a bilirubin greater than 2 mg/dL are not considered for resection. In patients who present with obstructive jaundice, resection is only considered after resolution of the jaundice using preoperative endoscopic or percutaneous biliary drainage.
Because there is a sliding scale of FLR volume minimums and liver quality, liver biopsy can be used to diagnose preexisting liver damage and evaluate whether this damage is acute, subacute, or chronic in nature (Fig. 10.4). Preoperatively, this can be easily done percutaneously. Or, if diagnostic laparoscopy is being performed for staging or for another minor procedure, a wedge biopsy can be done simultaneously. However, as simple as a biopsy may seem, two major problems must be recognized. First, the distribution of parenchymal diseases, such as fibrosis, steatosis, and chemotherapy-associated liver injury (CALI) such as sinusoidal obstruction syndrome (SOS) and steatohepatitis (SH), is heterogeneous, and thus a small liver biopsy can be associated with false-negative results. Secondly, these histopathologic diagnoses do not accurately predict postresection liver function, FLR regenerative capacity, and protection from postoperative hepatic insufficiency (PHI). Thus, while a positive liver biopsy may be helpful, caution should still be exercised if the biopsy is “normal” but the liver looks clinically damaged. Some parenchymal changes such as fatty liver can be grossly evaluated with cross-sectional imaging by comparing the tissue perfusion and attenuation to that of the spleen and abdominal fat. Many cirrhotic changes from portal hypertension are also readily apparent on imaging as aforementioned. Current work is being done to more precisely correlate computed tomography (CT) and magnetic resonance imaging (MRI) findings with pathologic parenchymal changes.
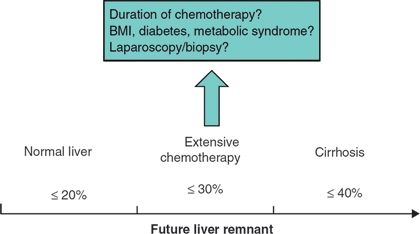
FIGURE 10.4 Diagram of the future liver remnant required for hepatectomy depending on the parenchymal quality and any preexisting liver injury. (BMI, body mass index).
Indocyanine green retention rate at 15 minutes (ICG R15) is used as a global measure of liver function. In this test, faster clearance (lower retention percentage at 15 minutes) implies better liver function and drainage. Used extensively in East Asia but less so in North and South America and Europe, a decision tree known as the Makuuchi criteria is used to evaluate livers with possible chronic disease before minor hepatectomy (usually for HCC). The qualitative cutoff values of ICG R15 may be less helpful in stratifying livers for major hepatectomy when compared to regeneration criteria such as those provided by PVE (see below). ICG R15 cutoffs for wedge resection and segmentectomy are 30% to 39% and 20% to 29%, respectively. Left hepatectomy or sectionectomy requires 10% to 19% retention or less. Finally, right or extended hepatectomy is considered only if ICG R15 is less than 10%. An important initial consideration in applying these criteria is that patients with ascites and/or elevated bilirubin (> 2 mg/dL) do not qualify for any type of resection irrespective of the ICG cutoffs.
PREOPERATIVE EVALUATION
Risk Factors for Complications
Complications specific to liver surgery include bleeding (both intraoperative and early postoperative), bile leak, and postoperative hepatic insufficiency (PHI or liver failure) with PHI-related mortality. The current authors previously defined PHI as a peak total bilirubin greater than 7 mg/dL, which has a sensitivity and specificity of greater than 93% and an odds ratio of 10.8 for predicting 90-day mortality. Acute PHI often leads to an irreparable slide toward liver failure–related mortality that continues well past 30 days. In regard to measuring surgical outcomes, one-third of posthepatectomy deaths occur between 30 and 90 days, so typical short-term metrics fail to fully capture late liver-related mortalities.
Predictors of major complications can be divided into three categories—medical comorbidities, laboratory abnormalities, and perioperative risk factors (including extent of hepatectomy). These parameters include low albumin, smoking, American Society of Anesthesiologists (ASA) class, elevated alkaline phosphatase, elevated partial thromboplastin time (PTT), extent of hepatectomy, prolonged operative time, and intraoperative or postoperative transfusions. The last three factors are associated with each other, and thus, surgeons should be cautious with patient selection when pairing major simultaneous operations with major hepatectomies. While not every risk factor is reversible, many are potentially modifiable in the preoperative period through optimization of medical issues and planning operations of lesser magnitude when oncologically practical.
Initial Assessment
The initial preoperative assessment of a patient requiring a liver resection takes into account three major factors—patient operability (performance status and comorbidities), liver quality (global function), and tumor extent (balance of oncologic resectability and FLR). An irreversible or unmodifiable issue with the patient or the liver precludes liver resection. The risks of liver resection and PHI are relatively unique, because the liver is the only abdominal visceral organ without which a patient cannot survive. Thus, unless a transplant is planned, enough functional parenchyma must be left behind, or the patient will inevitably die from liver failure.
Patient Operability
The term “operability” describes patient, not tumor, factors. Patients can be operable, inoperable, or borderline operable if they have potentially reversible comorbidities. Operability is dependent on a patient’s functional capacity (performance status) and underlying medical comorbidities. If both are good, then the patient is operable. If either is irreversibly poor, then the patient is inoperable. If functional or medical issues are potentially reversible, then the patient is considered borderline operable. The fact that grading performance status is part of the National Comprehensive Cancer Network (NCCN) preoperative assessment for hepatocellular carcinoma (HCC) highlights the importance of assessing patient operability. With aggressive prehabilitation, nutritional counseling, and/or medical optimization, a borderline operable patient may become an operable surgical candidate and has the potential to enjoy the same survival benefit from resection as a patient who was initially operable. If a patient is undergoing preoperative chemotherapy, this time period offers an opportunity to address the aforementioned issues. Even if preoperative therapy is not considered or not indicated, taking appropriate time to optimize patient physiology before surgery will not only decrease surgical complications but will also improve the clinical rescue rate of patients after major complications and decrease the postcomplication mortality rate.
Assessment and Optimization of Future Liver Remnant
The FLR is the predicted volume of remaining liver after resection. Sufficient size, synthetic function, biliary drainage, perfusion, and regenerative capacity are essential to avoid both early and late PHI. Evaluating the FLR’s volume is currently the most reliable approach to predict outcomes for patients who are candidates for major resection. Several methods for such evaluation have been described. At the University of Texas MD Anderson Cancer Center (MDACC), the estimated total liver volume (TLV) is calculated using a formula that relies on the linear correlation between the TLV and body surface area (BSA): TLV (in cm3) = − 794.41 + 1,267.28 × BSA (m2). The “standardized” FLR is then calculated as the ratio of the predicted FLR volume to the standardized TLV.
In a series of 301 patients without hepatic injury or chronic liver disease undergoing extended right hepatectomy, a standardized FLR of less than 20% was a risk factor for PHI and 90-day postoperative mortality. Generally, the required minimum standardized FLR is greater than 20% for normal livers, greater than 30% for livers with limited parenchymal injury (e.g., extended duration chemotherapy), and greater than 40% for permanently damaged livers (e.g., cirrhosis, Fig. 10.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree