Late Events and Long-Term Complications After Treatment for Testicular Cancer
Jan Oldenburg
Hege S. Haugnes
Sophie D. Fosså
INTRODUCTION
The combination of rising incidence of testicular cancer (TC), high cure rates (˜95% achieve 5-year survival), and the young age at diagnosis with a posttreatment life expectancy of 30 to 50 years increases the number of testicular cancer survivors (TCSs) extensively. Thereby, TCSs are considered an ideal population for survivorship research. Considerable efforts have been undertaken to elucidate long-term complications ranging from life-threatening diseases such as myocardial infarction or second cancers to subclinical impairment of physiologic organ function (1).
In addition to these treatment-related long-term complications, late events not representing treatment sequelae such as late relapses and contralateral TC may occur many years after discontinuation of uro-oncologic follow-up. In this chapter, we intend to give an overview about the most important longterm complications and late events in TCSs.
LATE EVENTS
Late Relapse
Late relapses are defined as recurrences at least 2 years after successful TC treatment and occur in approximately 3.2% and 1.4% of patients with nonseminoma and seminoma, respectively (2). The late relapse risk increases with the initial extent of disease and, most importantly, by inadequate initial treatment. TC metastases almost always affect retroperitoneal lymph nodes, which also represent the most common site of relapse as experienced by 270 of 521 (51.8%) of TC patients included into a pooled analysis (2). In patients with metastatic nonseminoma, late relapses are probably most effectively prevented by adequate postchemotherapy (PC) retroperitoneal lymph node dissection (RPLND) (3,4).
Late relapses may occur years after treatment, posing considerable challenges to the optimal follow-up time. In our population-based experience of 1,949 patients, all except one late relapse occurred within 10 years after treatment, and we recommend this time frame as a minimum follow-up schedule. The fear of relapse in TCSs, 5 to 20 years after successful treatment, was associated with psychosocial features, for example, being married, having children, high education, neuroticism, and not factors important for the risk of late relapse such as initial stage or histology (5). Thus, the policy to let patients decide on the length of follow-up appears questionable.
Detection of Late Relapse
During follow-up, late relapses may be detected by elevated tumor markers, radiological abnormalities, palpable masses, or other clinical findings. Approximately 50% and 25% of late relapsing patients have elevated a-fetoprotein or human chorionic gonadotropin, respectively. Typical clinical symptoms and findings comprise back-pain, fatigue, abdominal tumor, or a palpable mass (6).
Treatment of Late Relapse
Surgery is the most important treatment modality of a late relapse, but optimal treatment for the individual patient requires evaluation and planning by an experienced interdisciplinary team comprising urological surgeons, oncologists, pathologists, and radiologists. Additionally, vascular, thoracic, orthopedic, and neurosurgeons might need to be consulted. It has been shown that survival of poor prognosis patients is increased by treatment in high-volume centers (7). Most probably, this principle applies to late relapsing patients as well.
Achievement of a presalvage biopsy is of great importance as its result directs treatment: Complete resection is the only necessary and sufficient means to eradicate teratoma, which may be found in both initial seminoma and nonseminoma. Also in patients with somatic differentiated teratoma, a complete resection is the treatment of choice as salvage chemotherapy has only limited effect (8).
Seminoma
After radiation in seminoma CS I, relapses occur nearly exclusively outside the prior radiation field and are usually cured by cisplatin-based chemotherapy (2,9). Relapses following single agent carboplatin are mostly salvaged by cisplatin-based chemotherapy or by radiotherapy. Relapses after cisplatin-based chemotherapy in patients with advanced seminoma are very rare and alternative salvage chemotherapy should be pursued. Surgery should always be considered.
Nonseminoma
Surgery is considered the most important treatment modality (4,10,11). “Desperation surgery,” that is, removal of masses despite poor response to chemotherapy, may be curative (12).
Chemotherapy-naïve patients have the best chances to be cured (4,10,13), whereas the response to salvage chemotherapy in patients with prior chemotherapy exposure is low (10). The combination of paclitaxel, ifosfamide, and cisplatin (TIP) yields a complete response rate of 50% in late relapsing patients and represents the preferred regimen in these patients (14).
Survival
The reported cure rates have been increasing over the last three decades and range from 26% to 69% (6). Conceivably, survival was significantly higher in patients in whom surgical resection at late relapse was complete than those with incomplete resection, illustrating the importance of timely referral to centers with highly experienced surgeons (4). Furthermore, survival strongly depends on the histology of late relapse, whereas the
initial histology, that is, seminoma versus nonseminoma, is of minor importance. In our population-based experience, survival was 100% in patients who had a single teratoma, whereas only 50% survived a relapse with malignant germ cell tumor or teratoma with somatic transformation (Fig. 35.1) (6).
initial histology, that is, seminoma versus nonseminoma, is of minor importance. In our population-based experience, survival was 100% in patients who had a single teratoma, whereas only 50% survived a relapse with malignant germ cell tumor or teratoma with somatic transformation (Fig. 35.1) (6).
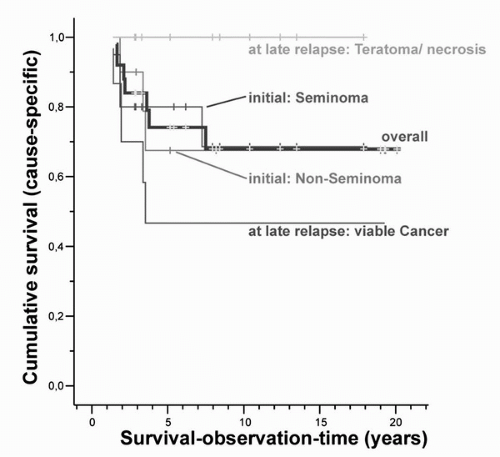 FIGURE 35.1. Cumulative survival according to initial and late-relapse histology. Survival for TCSs experiencing a late relapse, that is, recurrence of TC ≥ 2 years after successful treatment, according to initial histology, that is, seminoma or nonseminoma, and histology at relapse, that is, vital nonteratomatous germ cell tumor or teratoma with somatic transformation versus teratoma or necrosis. (From Oldenburg J, Alfsen GC, Waehre H, et al. Late recurrences of germ cell malignancies: a population-based experience over three decades. Br J Cancer 2006;94(6):820-827, with permission.) |
CONTRALATERAL TC
In a study based on the National Cancer Institute’s Surveillance, Epidemiology and End Results Program comprising 29,515 TCSs, the incidence of a contralateral TC at 1.9% is significantly higher than the incidence of a primary TC among age-matched controls (15).
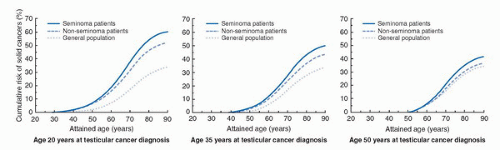 FIGURE 35.2. RR of a second solid cancer. Cumulative risk (%) of developing a second solid cancer for TCSs with seminoma and nonseminoma according to age at TC diagnosis and attained age. Risks beyond age 60 years for men diagnosed with TC at age 20 years, or beyond age 75 years for men diagnosed with TC at age 35 years represent extrapolation of estimated trends. (From Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors 4. J Natl Cancer Inst 2005;97(18):1354-1365, with permission.) |
Treatment corresponds to that of primary TC, but due to an increased awareness, advanced stages are rarely encountered and orchiectomy alone is most often sufficient. Before orchiectomy, semen cryopreservation should be offered. Testosterone substitution is mandatory. In case of very small tumors, organ sparing partial tumor enucleation may be performed at experienced centers, but required postsurgery radiation will cause infertility (16).
TREATMENT-RELATED COMPLICATIONS
Second Cancers
Non-germ cell malignancies may be induced by radiation, chemotherapy or both. Travis et al. (17) performed the most conclusive report on the risk of solid tumors by inclusion of 40,576 TCSs from 14 national cancer registries. Compared to the general population, the authors reported significantly increased relative risks (RRs) for cancer development among TCSs in the following organs: stomach, RR 4.0; pancreas, RR 3.6; mesothelioma, RR 3.4; bladder, RR 2.7; colon, RR 2.0; esophagus, RR 1.7; lung, RR 1.5. The risk of cancer development 40 years after treatment of 35-year-old seminoma and nonseminoma patients was with 36% and 31%, respectively, significantly higher than the 23% of the age-matched normal population (Fig. 35.2). The risk of second cancers increased ten years after treatment and remained so for several decades.
The RR of a solid non-germ cell tumor is approximately doubled after chemotherapy or radiotherapy, whereas combination of both techniques is associated with a threefold increased risk. Threshold values are, if existent, probably very low since even radiation doses from repeated abdominal CT examinations in surveillance patients, might account for second cancers in 1.8% and 1.2% of TCSs treated at an age of 18 -and 35 years, respectively. This indicates a particularly high vulnerability to radiation-induced carcinogenicity among the youngest patients (18).
Leukemias may be induced by etoposide and/or cisplatin, both of which are commonly applied in the treatment of TC (19). Kollmannsberger et al. (20) estimated the cumulative risk of leukemia as 0.5% and 2%, after cumulative etoposide doses of <2,000 mg/m2 and >2,000 mg/m2, respectively.
Treatment-induced leukemias mostly arise within one decade after treatment and the survival rates are very poor.
Solid second tumors, however, do usually occur at least 10 years after treatment discontinuation, and survival rates are not inferior to those observed in the patients developing a solid tumor without prior TC treatment (21).
Solid second tumors, however, do usually occur at least 10 years after treatment discontinuation, and survival rates are not inferior to those observed in the patients developing a solid tumor without prior TC treatment (21).
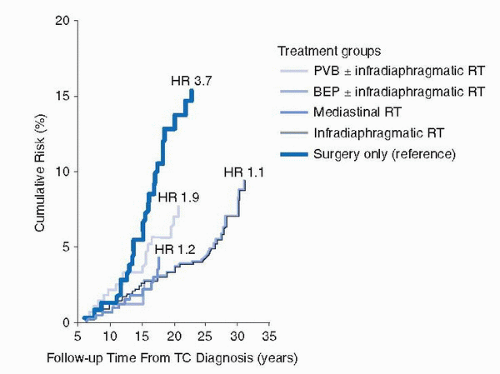 FIGURE 35.3. Risk of myocardial infarction according to treatment group. Risk of myocardial infarction. BEP, bleo+mycin, etoposide, and cisplatin; HR, hazard ratio; PVB, cisplatin, vinblastine, and bleomycin; RT, radiotherapy; TC, testicular cancer. (From van den Belt-Dusebout AW, Nuver J, de Wit R, et al. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol 2006;24(3):467-475, with permission.) |
Cardiovascular Disease
Mortality from cardiovascular disease (CVD) is higher in TCSs than in the general population (22,23). An increased incidence of CVD, both fatal and nonfatal, has been observed in men previously treated with cisplatin-based chemotherapy in comparison to men treated with surgery only (24,25). Treatment with cisplatin, vinblastine, bleomycin (CVB) was significantly associated with an 1.9-fold increased risk for myocardial infraction (MI), while cisplatin, etoposide and bleomycin (BEP) gave a nonsignificantly increased risk for MI at 1.2 in comparison to surgery only treated men (Fig. 35.3) (25).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








