Chapter 33 Larynx and Hypopharynx Cancer
Epidemiology and Etiology
The median age of patients presenting with larynx cancer is 65 years. Less than 4% of patients are younger than 45 years old. Laryngeal cancer remains predominantly a disease affecting men. The most recent data from the Surveillance, Epidemiology, and End Results (SEER) program (2003–2007)1 estimated an overall incidence of 3.4 cases per 100,000 people per year, a slight decrease of 0.4 cases per 100,000 compared with the previous 5 years of data.2 Comparisons reveal case incidences of 6.1 versus 1.3 in males and females, respectively. There is a higher incidence among blacks than among whites for both men and women.
Tobacco use is strongly associated with the development of cancer of the larynx, with the highest risk among active heavy smokers and an intermediate risk among ex-smokers.3,4 More than 95% of patients with laryngeal cancer have a history of tobacco use.5 Cigar and pipe smoking have also been associated with cancer of the larynx,4 but studies on this issue have been more controversial.3,6,7
Alcohol use is also associated with laryngeal cancer but is believed to act synergistically with tobacco rather than independently. It is unusual to see laryngeal cancer in nonsmoking patients with alcohol abuse histories.7 A history of heavy alcohol use is more strongly associated with supraglottic and hypopharyngeal cancers.4,8 Likewise, occupational exposures to asbestos,4,9,10 mustard gas, nickel, soot, and tars have been linked to laryngeal cancer, but generally a tobacco use history is also present.11 Several authors have evaluated the influence of diet on the development of larynx cancer and have found, while controlling for tobacco and alcohol use, a higher incidence among patients with vitamin- and nutrient-deficient diets.6,12,13
Attention has been directed at the influence of gastroesophageal reflux disease (GERD) on laryngeal diseases, including carcinomas. Three separate studies14,15,16 have described cohorts of nonsmoking patients with GERD and larynx cancer. Bacciu and colleagues17 compared 36 consecutive patients with no history of tobacco and alcohol consumption who developed laryngeal carcinoma to a group of 125 lifetime nonsmokers who were cancer free. They found a much higher prevalence of GERD among the patients with laryngeal cancer. It is believed that chronic irritation on the larynx from acid may predispose these patients to cancer. It is thought that if this cancer is seen in nonsmokers, the influence of GERD on the development of laryngeal cancer in smokers and alcohol users (who are at higher risk for GERD) may be very significant.
Human papillomavirus (HPV) has been causally linked to multiple cancers, including head and neck cancers,18,19 particularly cancers of the tonsil. HPV has also been demonstrated to be associated with laryngeal cancers, although studies are, in general, retrospective and the reported prevalence rates vary widely. The evidence for HPV having a role in laryngeal cancer is less obvious than in other malignancies, and its interaction with other carcinogens such as tobacco is unclear. As in cervical cancer in women and tonsillar cancer, HPV-16 is the most common type noted.
Hypopharyngeal cancers are less common than laryngeal tumors. The estimated incidence in the United States is 2500 cases per year. Etiologic risk factors are similar to those for laryngeal tumors,12 with a predominance among men and older individuals. This type of cancer is closely linked to tobacco and alcohol use, and the ties to heavy alcohol use seem stronger than those for laryngeal cancer.
Prevention and Early Detection
Prevention
The primary preventive methods taken to eliminate malignancies of the upper aerodigestive tract have come from public awareness that tobacco and alcohol are the major causative agents of these cancers. National public health measures have been directed at diminishing the prevalence of smoking and drinking. A byproduct of these policies may be a decline in the incidence of laryngeal and hypopharyngeal carcinomas. The most recent SEER data has identified a decrease in the incidence of larynx cancer over the past two decades (1988–2007).1 The annual percentage decrease over this time period is 2.6%.
Although government policies have been directed at diminishing carcinogenic etiologic agents for the general population, investigators have tried to identify high-risk groups in whom more direct measures can be taken. The major group identified consists of patients who have been cured of a cancer of the upper aerodigestive tract,20,21–23 particularly patients who have smoking or alcohol use histories.24 The incidence of second primary cancers of the upper aerodigestive tract ranges from 10% to 30%. The locations of these second cancers are evenly divided between the lungs, esophagus, and head and neck mucosal sites, including the larynx and hypopharynx.24
The high incidence of new cancers in this patient population has led investigators to develop programs designed to diminish the occurrence of second primary tumors. Hong and associates25 studied 13-cis-retinoic acid as a possible agent to prevent new cancers in patients with a history of head and neck malignancy. A randomized trial showed a 14% versus 31% incidence of second primary tumors in patients who received a relatively high dose of 13-cis-retinoic acid versus placebo.26 The Radiation Therapy Oncology Group (RTOG), in 2002, completed accrual to a trial testing chemoprevention with 13-cis-retinoic acid in a multi-institutional setting.27 Nearly 1400 patients with stage I or II cancer were accrued. The dose of 13-cis-retinoic acid was lower than that used by Hong and associates to ensure more compliance and less side effects. Unfortunately, the RTOG trial was negative and did not show any benefit to low dose isotretinoin in the prevention of second primary cancers. The RTOG study did show that the continuation of smoking had adverse effects on outcome and those who smoked were more likely to develop second cancers.
Papadimitralopoulou and colleagues28,29 investigated α-tocopherol, interferon-alfa, and isotretinoin in patients with laryngeal dysplasia and reported a 50% complete response rate at 12 months. The current thinking is that these agents may delay or prevent subclinical cancer from manifesting as clinical disease but are less likely to prevent cellular transformation. This three-drug combination is being tested in a phase III trial, but accrual has not been robust.
Early Detection
Similar to the issues surrounding prevention, early detection of laryngeal and hypopharyngeal cancers centers on targeting the population at highest risk for developing these carcinomas. Cancers of the larynx and hypopharynx affect roughly 15,000 Americans a year and therefore is not a large enough health problem to warrant screening of the general population. Some investigators have studied the role of screening a more focused population such as tobacco users who work at high-risk occupations and question the value of screening even a more limited population.30 However, Prout and colleagues31 argue that a primary care practitioner can, as part of a general evaluation, inquire about hoarseness in a patient from an at-risk population. If a positive response is obtained, the patient can be referred to an otolaryngologist for appropriate evaluations. The laryngeal carcinoma detection rate in this situation ranges between 3% and 4%.31,32
Pathology and Pathways of Spread
Pathology
Less frequently, the larynx and hypopharynx can give rise to variants of squamous cell carcinoma. The most common of these cancers is verrucous carcinoma, accounting for approximately 4% of all larynx cancers.33 They are classically slow-growing tumors with a gross warty appearance. A less common variant with numerous nomenclatures is squamous cell carcinoma with spindle cell features. As its name implies, along with typical squamous cells, carcinoma cells are spindle cells. The significance of these spindle cells is the subject of debate, because theories range from these cells being a benign reactionary process with little clinical significance to highly malignant elements with adverse outcome.34 Molecular evidence suggests the sarcomatoid carcinoma evolves from the conventional epithelium-type component and the sarcomatoid component has a malignant nature.35 Grossly, they can often present as large polypoid lesions that sometimes act as ball valves in the larynx. Basaloid squamous cell carcinoma and lymphoepithelioma of non-nasopharyngeal origin are rare tumors seen in numerous head and neck mucosal sites, including the larynx and hypopharynx.
The remaining 5% of larynx cancers are composed of neoplasms more commonly found in other locations. Salivary gland cancers, neuroendocrine tumors36 (including small cell carcinomas), sarcomas, and lymphomas have all been reported in the literature.37
Pathways of Spread
Primary Site and Regional Lymphatics
Larynx
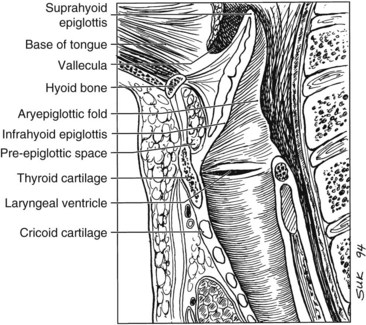
The larynx is divided into three regions: the supraglottis, glottis, and subglottis. The supraglottic larynx lies above the level where the mucosa of the upper surface of the true vocal cords turns upward to form the lateral wall of the ventricle. It consists of the false vocal cords, arytenoids, aryepiglottic folds, and infrahyoid and suprahyoid epiglottis. The glottic region by definition includes the true vocal cords and extends 0.5 cm inferiorly, and the subglottic region extends from there to the superior aspect of the trachea (Fig. 33-1).
Glottis
The mucosa of the true vocal cords has a sparse lymphatic supply. Thus, glottic carcinomas have a low propensity for lymphatic spread. The incidence of lymphadenopathy at diagnosis is approximately 5% for T1 and T2 lesions and approximately 20% for T3 and T4 tumors.38 The frequency of occult nodal involvement is also low. Byers and colleagues39 found microscopic nodal involvement in 9 of 57 patients who underwent elective nodal dissection for T3 or T4 vocal cord lesions; most frequently involved nodes were the upper jugular (level II), midjugular (level III), and paratracheal groups (level VI).
Supraglottis
The primary difference between supraglottic cancers and true glottic cancers is the likelihood of developing cervical nodal metastases. At diagnosis, 55% of patients with supraglottic cancers have clinically involved lymph nodes. Lymphatic vessels in the supraglottic larynx collect in channels that pass through the piriform sinuses to drain to nodes along the jugular chain, particularly the upper (level II) and midjugular (level III) lymph nodes. Lee and associates40 reported on the data of a subgroup of patients with intermediate-stage disease who underwent supraglottic laryngectomy with neck dissections. One third of patients had palpable nodes on presentation, and nearly an additional third of patients had pathologic nodal involvement.
Hypopharynx
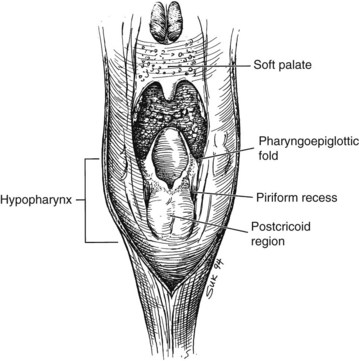
The hypopharynx is the inferior portion of the three divisions of the pharynx (Fig. 33-2). It extends from the hyoid bone superiorly to the cricoid inferiorly. Valleculae, pharyngoepiglottic fold, and lateral projections of aryepiglottic folds are considered the superior border separating the hypopharynx from oropharynx. Inferiorly, the hypopharynx ends at the cervical esophageal inlet. The hypopharynx is subdivided into three components: the pharyngeal walls, the piriform sinus, and the postcricoid pharynx. The hypopharyngeal walls are a continuation of the lateral and posterior oropharyngeal walls. The pair of piriform sinuses is created by the invagination of the larynx into the hypopharynx. They are conical (more truly pear shaped, hence the derivation of its name). Each sinus (or recess) consists of three walls. The medial wall is essentially the lateral aspect of the larynx; superiorly, it becomes the aryepiglottic fold. The lateral wall is a continuation of the lateral wall of the oropharynx. Anteriorly, the medial and lateral walls converge to form the narrow anterior wall. Superior is the base or vestibule formed by the rim of the three walls. Inferiorly, the three walls merge to form the apex.
Hypopharyngeal cancers also commonly present as nodal metastases. Lindberg41 reported a 75% incidence of nodal metastases in patients presenting to the M.D. Anderson Cancer Center (MDACC) with hypopharyngeal tumors. Level II and III nodes were most frequently involved, and bilateral lymphadenopathy was seen in 15% of patients. These tumors also have access to deep jugular and retropharyngeal lymph nodes.
Distant Metastases
The incidence of distant metastases from cancers of the head and neck and specifically laryngeal and hypopharyngeal cancers is low and is generally thought to be less than 10%. An often-referenced study by Crile in 190642 reported an incidence of 1% distant metastases in 4500 patients with epidermoid cancers of the head and neck. In subsets of patients with head and neck squamous cell cancers, patients with glottic tumors usually have the lowest rates of distant metastases whereas patients with hypopharyngeal carcinomas often have the highest rates.
Merino and colleagues43 analyzed the incidence of distant disease in over 5000 patients with head and neck squamous cell carcinomas treated from 1948 through 1967. Among patients with control of disease above the clavicle, the incidence of distant failure was 1%, 13%, and 23% for patients with carcinomas of the true vocal cords, supraglottic larynx, and hypopharynx, respectively. Similarly, Marks and associates44 found that 23% of patients with piriform sinus cancers developed distant metastases, with a higher rate among patients who underwent total laryngectomies as part of their therapy. The incidence of metastases is associated with stage of disease, because the clinical incidence of metastases is approximately 20% in patients with stage IV disease.
The sites of presentation of distant metastases are similar for cancers of the larynx and hypopharynx.43 The lungs are the most common site and are the first site of presentation in nearly 60% of patients. Bones are the next most common site, because 20% of patients with distant disease develop osseous metastases. Liver metastases are common in autopsy studies, but clinical liver metastases develop in only 10% of patients with hematogenous spread of disease from the larynx and hypopharynx.45 Spread to mediastinal lymph nodes, the brain, or other organs is very uncommon.
Molecular Biology
A general view is that tumorigenesis of head and neck malignancies occurs because of a combination of factors. Carcinogen exposure (primarily tobacco) results in genetic damage. However, not all smokers develop cancer. The genetic damage in individuals varies based on the degree of exposure of the offending agent as well as the individual’s inherent sensitivity to genetic damage. The latter can be tested indirectly in individuals using an assay quantifying chromosomal breakage induced by in-vitro exposure to bleomycin. Patients with upper aerodigestive tract malignancies were compared with healthy controls. Mutagen-sensitive individuals had a higher likelihood of having a cancer, and mutagen-sensitive smokers were at the highest risk.46
A field of mucosa is placed at risk for developing carcinoma by the exposure and sensitivity described earlier, but it requires a number of events to develop a frank cancer. The concept of field cancerization was first described in 1953 by Slaughter and associates.47 These researchers found widespread microscopic abnormalities in “normal” mucosa adjacent to tumor obtained from resected specimens. These abnormalities ranged from hyperplasia to areas of carcinoma both invasive and in situ away from the known resected cancer. Califano and colleagues48 proposed a genetic progression model in which the local clinical phenomenon of field cancerization involves the expansion and migration of clonally related preneoplastic cells.
The molecular events leading to the development of carcinomas are believed to occur by a multistep process and have been described for other cancers.49 This theory is applicable to head and neck cancers and appears consistent with the pathologic findings of field cancerization. Genotypic alterations in histologically normal epithelium adjacent to invasive carcinomas have been described. Voravud and associates50 found increased chromosomal polysomies in normal mucosa of smokers with invasive carcinomas but not in mucosa of healthy nonsmoking volunteers. The most frequent findings in premalignant tissue are deletions of one of the two alleles at chromosomes 3p and 9p21.51 These regions harbor tumor suppressor genes and thus may be involved with malignant transformation. It has also been observed that telomerase is activated in head and neck squamous carcinoma and dysplasia and may be an early event in the tumorigenic process.51
TP53 is a tumor suppressor gene. A mutation in TP53 is regarded as the most common related genetic change in human cancers.52 The normal TP53 protein has a short half-life, so its detection is thought to represent TP53 mutation. Shin and colleagues53 found TP53 expression in normal tissue and premalignant lesions adjacent to head and neck tumors but not in normal tissues from normal control patients. Numerous investigators have found overexpression of TP53 protein in laryngeal carcinomas, typically in approximately half the tumors studied.53,54 Alterations in TP53 are thought to be an important step early in the carcinogenic process but not a necessary step for all laryngeal and hypopharyngeal tumors. The finding of this protein in laryngeal specimens is also unclear, because, although there are diverging study results, most investigators have not found a prognostic value to the finding of TP53 protein in larynx tumor specimens.54–57
Other cellular changes in laryngeal carcinogenesis involve epidermal growth factor receptor (EGFR), CCND1 (formerly cyclin D1), and CDKN2A. Weichselbaum and colleagues58 described elevations in EGFR expression in head and neck tumor cell lines. In an analysis of premalignant and malignant head and neck tumor specimens, EGFR levels correlated with increasing severity of dysplasia.59 Furthermore, Shin and colleagues60 found elevated EGFR levels in premalignant tissues but dramatic up-regulation in invasive carcinoma specimens. EGFR was amplified in DNA from 29% of sampled hypopharyngeal cancers, and it is overexpressed in at least 80% of head and neck cancers. This overexpression has been demonstrated to be an independent prognosticator in head and neck cancer.59
Clinical Manifestations, Patient Evaluation, and Staging
Patient Evaluation
Laryngeal Cancer
Clinical evaluation of laryngeal cancer includes indirect laryngoscopy with a mirror that is frequently supplemented by fiberoptic endoscopy (Table 33-1). Similar to other tumors in the head and neck, the examiner assesses the tumor size, morphology, infiltration (defect, distortion) of adjacent structures, and vocal cord mobility. It is important to palpate the base of the tongue to determine direct invasion from the supraglottic larynx and to look for indirect signs of pre-epiglottic space invasion such as fullness of the vallecula or ulceration of the infrahyoid epiglottis. A direct laryngoscopy is often the final step in evaluation. It is needed to further outline the disease extent and, in particular, to obtain biopsy specimens for tissue diagnosis.
TABLE 33-1 Evaluation of Patients with Suspected Laryngeal or Hypopharyngeal Cancer
* In patients with more advanced disease, these tests may augment the workup for metastatic disease; PET and barium studies may help better define the inferior extent of advanced hypopharyngeal cancers.
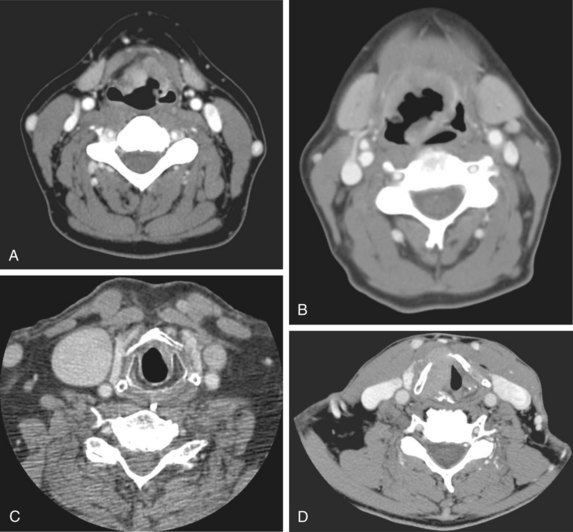
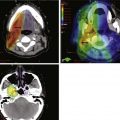
Radiologic studies are indicated when there is the suggestion of deep infiltration. CT (Fig. 33-3) or MRI is useful for the assessment of the pre-epiglottic space, tongue base, paraglottic region, and subglottis (which is sometimes difficult to evaluate from above with the mirror or fiberoptic scope). These images may help differentiate direct primary tumor extension to the soft tissue of the neck from nodal involvement. Anterior commissure lesions may have subtle thyroid cartilage invasion only detectable by CT.
Hypopharyngeal Cancer
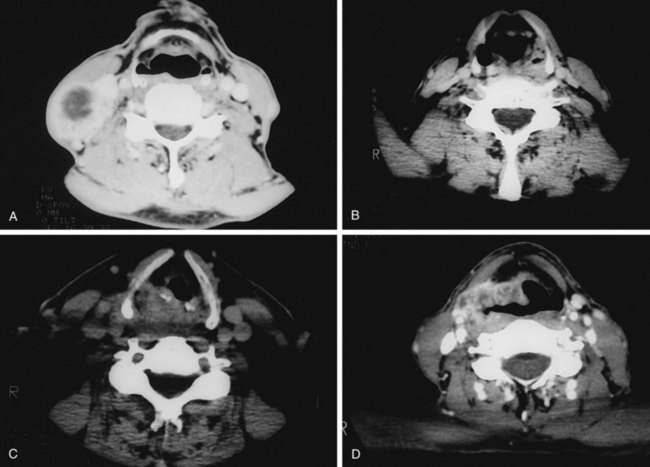
Evaluation of hypopharyngeal tumors is similar to that of larynx cancers. Because these lesions are typically at an advanced stage, it is not uncommon to palpate disease in the neck by direct extension. The thyroid click may be lost owing to anterior displacement of the larynx by a posteriorly located (particularly postcricoid) lesion. Phonation may provide better visualization of the piriform sinus on indirect mirror and fiberoptic examination. If unsuccessful, a Valsalva maneuver may open the sinus. Deep infiltrative lesions in the apex may be hard to see but are suspected by either pooling of secretions or arytenoid edema. Assessment of laryngeal mobility is important in medial wall lesions because they can invade directly into the laryngeal framework. In addition to CT (Fig. 33-4), combined 18F-fluorodeoxyglucose-labeled positron emission tomography and CT (FDG-PET/CT) may be helpful in defining tumor extent, particularly the inferior border where subtle changes in the inferior hypopharynx and superior cervical esophagus may be hard to identify solely on anatomic images.
Staging
Larynx
Tumors are staged using the American Joint Committee for Cancer (AJCC) TNM system27 (Table 33-2). The system is currently in its seventh iteration, most recently revised in 2010. The system classifies/categorizes larynx cancer primary tumors (T) by sites of extension and cord mobility but not size. For glottic tumors, stage T1 is disease limited to the vocal cord(s). The AJCC staging system further divides T1 glottic tumors into T1a for tumors confined to one true vocal cord and T1b for tumors involving both vocal cords. In general, this subdivision is more useful for communication and may influence the treatment decision (i.e., limited surgery vs. RT) but does not have a strong impact on prognosis. T2 glottic tumors have either supraglottic or subglottic extension and/or impaired mobility. Although not defined by the AJCC, T2 glottic lesions can be subdivided into T2a for normal mobility and T2b for impaired mobility. This subdivision is not uniformly used, however, because there is no general agreement that treatment outcomes differ among the substages.
TABLE 33-2 American Joint Committee on Cancer Staging of Laryngeal and Hypopharyngeal Cancers
| Glottis | |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to the vocal cord(s) with normal mobility |
| T2 | Tumor extends to supraglottis and/or subglottis and/or with impaired cord mobility |
| T3 | Tumor limited to the larynx with vocal cord fixation and/or invades paraglottic space and/or minor thyroid cartilage erosion |
| T4a | Tumor invades through the thyroid cartilage and/or invades tissues beyond the larynx |
| T4b | Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
| Supraglottis | |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to one subsite of the supraglottis with normal vocal cord mobility |
| T2 | Tumor invades mucosa of more than one subsite of the supraglottis or glottis or adjacent site outside the glottis, without fixation of the larynx |
| T3 | Tumor limited to the larynx with vocal cord fixation and/or invades any of the following: the pre-epiglottic space, the postcricoid area, and/or minor thyroid cartilage erosion |
| T4a | Tumor invades through the thyroid cartilage and/or invades tissues beyond the larynx |
| T4b | Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
| Hypopharynx | |
| T1 | Tumor limited to one subsite of hypopharynx and 2 cm or less in greatest dimension |
| T2 | Tumor invades more than one subsite of hypopharynx or an adjacent site or measures between 2 and 4 cm in greatest dimension, without fixation of hemilarynx |
| T3 | Tumor measures greater than 4 cm or with fixation of hemilarynx |
| T4a | Tumor invades thyroid/cricoid cartilage, hyoid bone, thyroid gland, esophagus, or central compartment soft tissue |
| T4b | Tumor invades prevertebral fascia, encases carotid artery, or involves mediastinal structures |
| Nodal Involvement (N) | |
| Nx | Nodes cannot be assessed |
| N0 | No clinically positive node |
| N1 | Single clinically positive ipsilateral node 3 cm or less in diameter |
| N2 | Single clinically positive ipsilateral node more than 3 cm, but not more than 6 cm in diameter or multiple clinically positive ipsilateral or bilateral or contralateral nodes, none more than 6 cm in diameter |
| N2a | Single clinically positive ipsilateral node more than 3 cm, but not more than 6 cm in diameter |
| N2b | Multiple clinically positive ipsilateral nodes, none more than 6 cm in diameter |
| N2c | Bilateral or contralateral lymph node, none more than 6 cm in greatest dimension |
| N3 | Metastases in a lymph node more than 6 cm in greatest dimension |
| Stage Groupings | |
| Stage I | T1N0M0 |
| Stage II | T2N0M0 |
| Stage III | T3N0M0 |
| T1-3N1 | |
| Stage IVA | T4aN0-1M0 |
| T1-4aN2M0 | |
| Stage IVB | T4bN0-3M0 |
| T1-4bN3M0 | |
| Stage IVC | T1-4N0-3M1 |
From Edge SB, Byrd DR, Compton C, et al, editors: AJCC Cancer Staging Handbook, ed 7. New York, 2010, Springer, pp 33-57. Used with permission of the American Joint Committee on Cancer.
Two significant revisions were made to the sixth edition of the staging system.61 Historically, the definition of T3 glottic disease has been fixation of the vocal cord. The sixth edition expanded on this definition and included paraglottic space invasion or minor thyroid cartilage invasion. Previous editions had defined T4 disease as invasion through the thyroid cartilage, but a common misinterpretation was to include any thyroid cartilage invasion into the T4 category. The new definition was made to clarify this point. The significance of this distinction is in the era of larynx preservation (see later); many still think that the presentation of invasion through the cartilage into the extralaryngeal tissues precludes a nonsurgical approach, but patients presenting with minimal invasion into the inner cortex may have disease suitable for larynx preservation.
Hypopharynx
T category for hypopharyngeal tumors is also based on sites of involvement and larynx motion (an indirect measurement of disease extension). The fifth edition of this staging classification had a significant modification to also include the size of the tumor in staging.62 This change addressed a major deficiency in prior systems, because the older systems tended to understage hypopharynx tumors relative to other head and neck mucosal cancers. The hypopharyngeal T category still does not reflect morphology, which remains an important criterion for selection of local therapies, because exophytic tumors in this site are usually selected more frequently than infiltrative lesions for organ-preserving therapies. The sixth edition made one additional modification. As described for laryngeal cancers, resectable T4a and unresectable T4b categories were defined. T4a hypopharyngeal disease can invade thyroid or cricoid cartilages, hyoid bone, thyroid gland esophagus, or central compartment, while T4b disease invades prevertebral fascia, encases carotid artery, or involves mediastinal structures. The newest edition now defines T4a and T4b as moderately advanced and very advanced.
Regional Classification/Categorizaton
The nodal (N) category is uniform for all head and neck cancer, including laryngeal and hypopharyngeal tumors. No changes were made in the seventh edition. The details of T and N categories for laryngeal (glottic and supraglottic) and hypopharyngeal cancers are summarized in Table 33-2.
Primary Therapy
Early Carcinomas
T1 and T2 Glottic Tumors
There are several difficulties in comparing the results of various treatment options. Nearly all series are retrospective single-institutional studies. It is thus difficult to ascertain if small differences in control rates between series are real. Mendenhall and colleagues63 address this issue by defining the surgical procedure that would have been recommended in their irradiated patients. They found that the surgical treatment option would have required total laryngectomy, rather than partial voice-conserving procedures, in 10% of patients with T1 disease and 55% of those with T2 disease. However, the supracricoid laryngectomy, a procedure that removes an entire circumferential portion of the larynx and subsequently reconstitutes the larynx, has largely been developed during the past decade.64 Many patients described by Mendenhall and colleagues in 1993 deemed suitable only for total laryngectomy may now be managed with this voice-preserving surgery. Olsen65 states that it is very unusual for disease that does not fix the arytenoids to require total laryngectomy. Although this is possibly true from a technical standpoint, many larynx cancer patients are older, have long histories of tobacco exposure, and medically may not be suitable for partial laryngeal surgery other than excisions of small T1 lesions on the midcord.
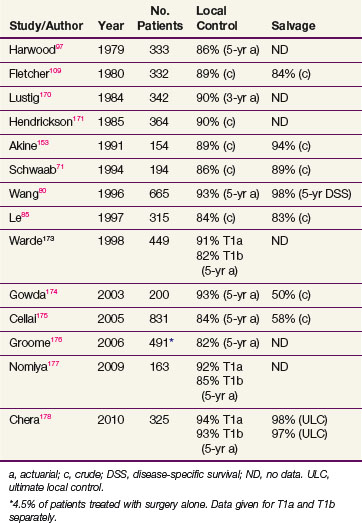
In general, T1 tumors can be treated effectively with laser excision,66 laryngofissure,67 partial laryngectomy,68,69 or irradiation. Table 33-3 summarizes the results of RT. Local control rates of RT range from 85% to 95%, and ultimate control rates after salvage surgery for recurrences are greater than 95%. Surgical salvage for the uncommon local relapse often requires a total laryngectomy, but an occasional patient can have a lesion amenable to a voice-conserving partial laryngectomy.70–74
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree