Laryngeal Cancer
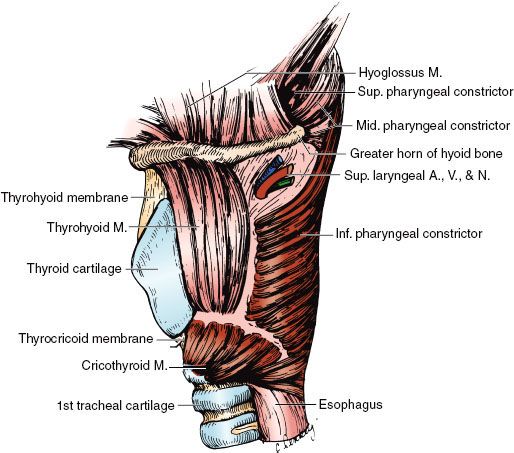
FIGURE 47.1. Diagrammatic sagittal section of the larynx. (Redrawn from Clemente CD. Anatomy: a regional atlas of the human body. Philadelphia: Lea & Febiger, 1975. Copyright Urban & Schwarzenberg, Munich, Germany, 1975.)
 ANATOMY
ANATOMY
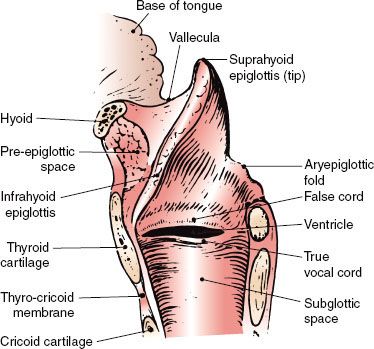
The larynx is divided into the supraglottis, glottis, and subglottis. The supraglottis consists of the epiglottis, false vocal cords, ventricles, aryepiglottic folds, and the arytenoids. The glottis includes the true vocal cords and the anterior commissure. The subglottis is located below the vocal cords (Figs. 47.1 and 47.2).1
The lateral line of demarcation between the glottis and supraglottic larynx is the apex of the ventricle. The demarcation between the glottis and subglottis is ill defined, but the subglottis is considered to extend from a point 5 mm below the free margin of the vocal cord to the inferior border of the cricoid cartilage or 10 mm below the apex of the ventricle.
The vocal cords vary from 3 to 5 mm in thickness and terminate posteriorly with their attachment to the vocal process. The posterior commissure is the mucosa between the arytenoids.
The shell of the larynx is formed by the hyoid bone, thyroid cartilage, and cricoid cartilage; the cricoid cartilage is the only complete ring. The more mobile interior framework is composed of the heart-shaped epiglottis and the arytenoid, corniculate, and cuneiform cartilages. The corniculate and cuneiform cartilages produce small, rounded bulges at the posterior end of each aryepiglottic fold.
The thyroid and the cricoid cartilages and a portion of the arytenoid cartilage are hyaline cartilage and may partially ossify with age, particularly in men. The epiglottis is elastic cartilage; ossification does not occur, and even focal calcification is rare.2
The external laryngeal framework is linked together by the thyrohyoid, the cricothyroid, and the cricotracheal ligaments or membranes (Figs. 47.3 and 47.4).1
The epiglottis is joined superiorly to the hyoid bone by the hyoepiglottic ligament. The epiglottis is joined to the thyroid cartilage by the thyroepiglottic ligament at a point just below the thyroid notch and above the anterior commissure. The arrangement of the ligaments that connect the cricoid and arytenoid cartilages and form the vocal ligaments, which are part of the true vocal cords, is shown in Figure 47.2B.1 The conus elasticus (cricovocal ligament) is the lower portion of the elastic membrane that connects the inferior framework. It connects the upper surface of the cricoid, the vocal process of the arytenoid, and the lower thyroid cartilage; its free border is thickened into the vocal ligament.
The vocal ligaments and muscles attach to the vocal process of the arytenoid posteriorly and the thyroid cartilage anteriorly. The intrinsic muscles of the larynx, which primarily control the movement of the cords, are presented in Figures 47.2 and 47.3.1 The extrinsic muscles are concerned primarily with swallowing. The cricothyroid muscle produces tension and elongation of the vocal cords and is innervated by the superior laryngeal nerve (Fig. 47.4).1
The pre-epiglottic and paraglottic fat spaces are essentially one contiguous space lying between the external framework of the thyroid cartilage and hyoid bone and the inner framework of the epiglottis and intrinsic muscles. Lam and Wong3 showed that there are thin membranous septa between the paraglottic and pre-epiglottic spaces that are capable of holding a tumor in check to a limited degree. The space is traversed by blood and lymphatic vessels and nerves. Because few capillary lymphatics arise in this area, invasion of the fat space should only indirectly be associated with lymph node metastases. The fat space is limited by the conus elasticus inferiorly, the thyroid ala, the thyrohyoid membrane, the hyoid bone anterolaterally, the hyoepiglottic ligament superiorly, and the fascia of the intrinsic muscles on the medial side. Posteriorly, it is adjacent to the anterior wall of the pyriform sinus.
The laryngeal surface of the epiglottis and the free margin of the vocal cords are squamous epithelium, and the remainder is usually pseudostratified ciliated columnar epithelium. Beneath the epithelium of the free edge of the vocal cord is the lamina propria, which can be divided into three layers. There is no true submucosal layer along the free margin of the vocal fold.4 The laryngeal arteries are branches of the superior and inferior thyroid arteries.
The intrinsic muscles of the larynx are innervated by the recurrent laryngeal nerve. The cricothyroid muscle—an intrinsic muscle responsible for tensing the vocal cords—is supplied by a branch of the superior laryngeal nerve; isolated damage to this nerve causes a bowing of the true vocal cord, which continues to be mobile, but the voice may become hoarse.
The supraglottic structures have a rich capillary lymphatic plexus; the trunks pass through the pre-epiglottic space and the thyrohyoid membrane and terminate mainly in the subdigastric (level II) lymph nodes; a few drain to the middle internal jugular chain (level III) lymph nodes.
There are essentially no capillary lymphatics of the true vocal cords; as a result, lymphatic spread from glottic cancer occurs only if tumor extends to supraglottic or subglottic areas.
The subglottic area has relatively few capillary lymphatics. The lymphatic trunks pass through the cricothyroid membrane to the pretracheal (Delphian) lymph nodes in the region of the thyroid isthmus. The subglottic area also drains posteriorly through the cricotracheal membrane, with some trunks going to the paratracheal (level VI) lymph nodes and others continuing to the inferior jugular (level IV) chain.
FIGURE 47.2. A: Cross section of the larynx at the level of the vocal cords. B: Framework of the larynx. (Redrawn from Clemente CD. Anatomy: a regional atlas of the human body. Philadelphia: Lea & Febiger, 1975. Copyright Urban & Schwarzenberg, Munich, Germany, 1975.)
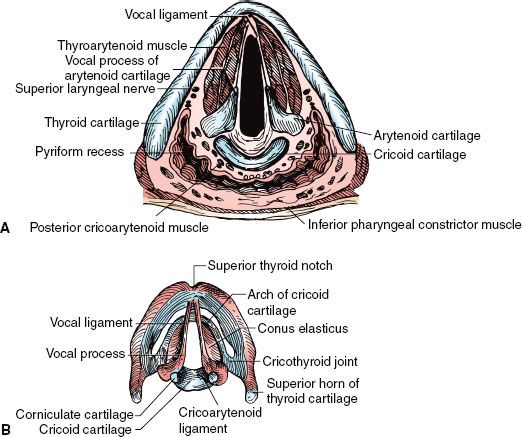
FIGURE 47.3. Diagram of the coronal view of the larynx. (Redrawn from Clemente CD. Anatomy: a regional atlas of the human body. Philadelphia: Lea & Febiger, 1975. Copyright Urban & Schwarzenberg, Munich, Germany, 1975.)
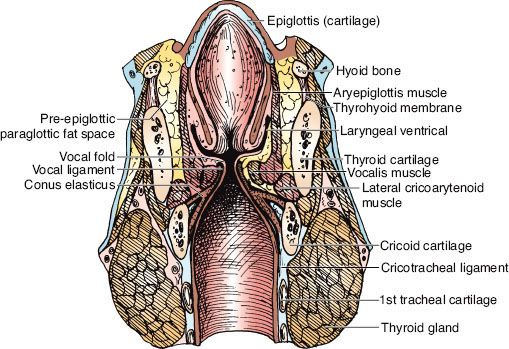
FIGURE 47.4. External view of the larynx. (Redrawn from Clemente CD. Anatomy: a regional atlas of the human body. Philadelphia: Lea & Febiger, 1975. Copyright Urban & Schwarzenberg, Munich, Germany, 1975.)
 EPIDEMIOLOGY AND RISK FACTORS
EPIDEMIOLOGY AND RISK FACTORS
Cancer of the larynx represents about 2% of the total cancer risk and is the most common head and neck cancer (skin excluded). In 2010 in the United States, there were approximately 12,720 new cases of cancer of the larynx (10,110 men and 2,610 women) and about 3,600 deaths from laryngeal cancer.5 Based on 1973–1998 U.S. data, at diagnosis, about 51% of the cases remain localized, 29% have regional spread, and 15% have distant metastases.6 The ratio of glottic to supraglottic carcinoma is approximately 3:1.
Cancer of the larynx is strongly related to cigarette smoking. The risk of tobacco-related cancers of the upper alimentary and respiratory tracts declines among former smokers after 5 years and is said to approach the risk of nonsmokers after 10 years of abstention.7 The role of alcohol in provoking laryngeal cancer remains unclear.8 Some evidence exists that heavy marijuana smoking may be associated with laryngeal cancer in young patients.
 PATTERNS OF SPREAD
PATTERNS OF SPREAD
Local Spread
Although supraglottic and glottic lesions tend to remain confined to their original compartments, there is no anatomic barrier to growth from one area to the next. Glottic lesions tend to be slow growing, but as they increase in size, they extend to the supraglottic and subglottic areas. Supraglottic lesions do not often start near the vocal cords. Involvement of the cords on their external epithelial surface is a late phenomenon, but submucosal extension by way of the paraglottic space occurs earlier.
The fat space is an important avenue of submucosal tumor spread for infrahyoid epiglottis, false cord, and true vocal cord lesions. As the false cord and the true vocal cord lesions penetrate anteriorly and laterally, they quickly encounter the tough perichondrium of the thyroid cartilage and may eventually be shunted by the conus elasticus (lateral cricothyroid membrane) out of the larynx via the cricothyroid space. Thyroid cartilage invasion usually occurs in the ossified section of the cartilage, commonly in the region of the anterior commissure tendon or the junction of the anterior one-fourth and the posterior three-fourths of the thyroid lamina.9
Fixation of the vocal cord from laryngeal cancer is usually caused by invasion or destruction of the vocal cord muscle, invasion of the cricoarytenoid muscle or joint, or, rarely, invasion of the recurrent laryngeal nerve. Perineural spread is uncommon.
Supraglottic Larynx
Suprahyoid Epiglottis
A lesion of the suprahyoid epiglottis may produce a huge exophytic mass with little tendency to destroy cartilage or spread to adjacent structures. Other lesions may infiltrate the tip and destroy cartilage. The destructive lesions tend to invade the vallecula and pre-epiglottic space, the lateral pharyngeal walls, and the remainder of the supraglottic larynx.
Infrahyoid Epiglottis
Lesions of the infrahyoid epiglottis tend to produce irregular tumor nodules and simultaneously invade the porous epiglottic cartilage and thyroepiglottic ligament into the pre-epiglottic fat space and extend toward the vallecula and base of the tongue. The thick hyoepiglottic ligament is an effective tumor barrier. However, the tumor may present in the vallecula and base of tongue without involving the suprahyoid epiglottis.
Lesions of the infrahyoid epiglottis grow circumferentially to involve the false cords, aryepiglottic folds, medial wall of the pyriform sinus, and the pharyngoepiglottic fold. Invasion of the anterior commissure and cords and anterior subglottic extension usually occur only in advanced lesions. Infrahyoid epiglottic lesions that extend onto or below the vocal cords are at a high risk for thyroid cartilage invasion, even if the cords are mobile.10
False Cord
Early false cord carcinomas, which are usually submucosal with little exophytic component, are difficult to delineate accurately. They involve the paraglottic fat space early in their development and may spread a considerable distance beneath the mucosa without producing physical signs. These carcinomas extend to the perichondrium of the thyroid cartilage quite early, but cartilage invasion is a late phenomenon. Extension to the lower portion of the infrahyoid epiglottis and invasion of the pre-epiglottic space are common. Submucosal extension involves the true vocal cord, which may appear normal. Vocal cord invasion is often associated with thyroid cartilage invasion. Submucosal extension to the medial wall of the pyriform sinus occurs early.
Aryepiglottic Fold/Arytenoid
Early lesions of the aryepiglottic fold/arytenoid are usually exophytic. It may be difficult to decide whether the lesion started on the medial wall of the pyriform sinus or on the aryepiglottic fold. As the lesions enlarge, they extend to adjacent sites and eventually cause fixation of the larynx, which is usually a result of involvement of the cricoarytenoid muscle or joint or, rarely, invasion of the recurrent laryngeal nerve. Computed tomography (CT) may distinguish the cause of fixation. Advanced lesions invade the thyroid, epiglottic, and cricoid cartilages and eventually invade the pyriform sinus and postcricoid area.
Glottic Larynx
Most lesions of the true vocal cord begin on the free margin and upper surface of the cord. When diagnosed, about two-thirds are confined to the cords, usually one cord. The anterior portion of the cord is the most common site. Anterior commissure involvement, which is common, is said to occur when no tumor-free cord can be seen anteriorly; if the lesion crosses to the opposite cord, anterior commissure invasion is certain. Small lesions isolated to the anterior commissure account for only 1% to 2% of cases. Extension to the posterior commissure is uncommon, occurring only in advanced lesions.
Tumors at the anterior commissure may extend anteriorly via the anterior commissure tendon (Broyles’ ligament)11 into the thyroid cartilage. Kirchner,12 using whole-organ sections, showed that such extension is unusual unless the tumor extends off the vocal cord onto the base of the infrahyoid epiglottis and suggested that the tendon serves as more of a barrier than an avenue of tumor spread. Early subglottic extension is also associated with involvement of the anterior commissure, and tumor may grow through the cricothyroid membrane.
Lesions that arise on the posterior half of the vocal cord tend to extend along the submucosa toward the medial side of the vocal process and invade the cricoarytenoid joint and posterior commissure; this spread is difficult to appreciate by clinical examination.
Subglottic extension may occur by simple mucosal surface growth, but it more commonly occurs by submucosal penetration beneath the conus elasticus. One centimeter of subglottic extension anteriorly or 4 to 5 mm of subglottic extension posteriorly brings the border of the tumor to the upper margin of the cricoid, exceeding the anatomic limits for conventional hemilaryngectomy. Lesions may spread beneath the epithelium along the length of the vocal cord within Reinke’s space.13
As vocal cord lesions enlarge, they extend to the false cord, vocal process of the arytenoid, and subglottis. Infiltrative lesions invade the vocal ligament and muscle and eventually reach the paraglottic space and the perichondrium of the thyroid cartilage. Advanced glottic lesions eventually penetrate through the thyroid cartilage or via the cricothyroid space to enter the neck, where they may invade the thyroid gland. Lesions involving the anterior commissure often exit the larynx via the cricothyroid space after they extend subglottically.13
A fixed cord that is associated with a lesion having <1 cm of subglottic extension and no false cord involvement does not ordinarily indicate invasion of the thyroid cartilage.12 If the false cord is also involved, cartilage invasion is likely.
Subglottic Larynx
Subglottic cancers are rare. Most involve the inferior surface of the vocal cords by the time they are diagnosed, so it is difficult to know whether the tumor started on the undersurface of the vocal cord or in the true subglottic larynx. Because early diagnosis is uncommon, most lesions are bilateral or circumferential at discovery. They involve the cricoid cartilages in the early stage because there is no intervening muscle layer. Partial or complete fixation of one or both cords is common; misdiagnosis or diagnostic delay is frequent.
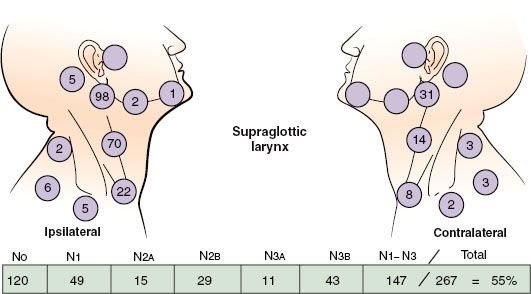
FIGURE 47.5. Nodal distribution on admission, MD Anderson Cancer Center, 1948–1965. (From Lindberg RD. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 1972;29:1446–1449; with permission.)
Lymphatic Spread
The location and stage of neck nodes detected on admission for previously untreated patients with squamous cell carcinoma of the supraglottic larynx are given in Figure 47.5.14 The disease spreads mainly to the level II nodes. The level Ib nodes are rarely involved, and there is only a small risk of level V lymph node involvement. The incidence of clinically positive nodes is 55% at the time of diagnosis; 16% are bilateral.14 Elective neck dissection shows pathologically positive nodes in 16% of cases; observation of initially node-negative necks eventually identifies the appearance of positive nodes in 33% of cases.15,16 Spread to the pyriform sinus, vallecula, and base of the tongue increases the risk of lymph node metastases. The risk of late-appearing contralateral lymph node metastasis is 37% if the ipsilateral neck is pathologically positive, but the risk is unrelated to whether the nodes in the ipsilateral neck were palpable before neck dissection.
The incidence of clinically positive lymph nodes at diagnosis for vocal cord carcinoma approaches zero for T1 lesions and is <2% for T2 lesions.17 The incidence of neck metastases increases to 20% to 30% for T3 and T4 lesions. Supraglottic spread is associated with metastasis to the level II nodes. Anterior commissure and anterior subglottic invasion are associated with involvement of the midline pretracheal lymph node (level VI).
Lederman18 reported a 10% incidence of positive lymph nodes in 73 patients with subglottic carcinoma.
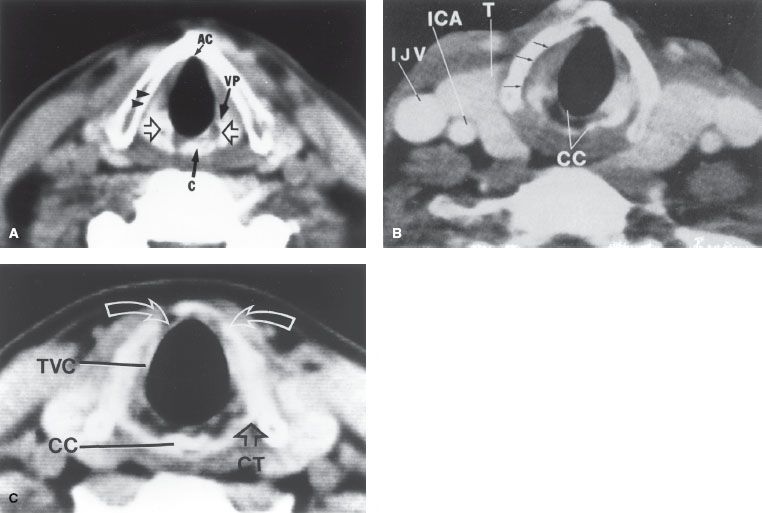
FIGURE 47.6. A: Normal CT anatomy of the midplane of the true vocal cords. Open arrows indicate arytenoid cartilages. The top of the cricoid cartilage (C) is partially visualized at this level. The vocal process (VP) of the left arytenoid cartilage is demonstrated. A narrow, low-density plane is seen between the right true vocal cord and the thyroid lamina (arrowheads); this is the inferior part of the paraglottic fat space. Notice the complete lack of tissue at the anterior commissure (AC). Any tissue density here should be considered abnormal. B: Normal CT anatomy just below the midplane of the vocal cords. Arrows indicate low-density lower paraglottic fat space. The fibrofatty tissue in this space facilitates separation of the vocal cord and the adjacent thyroid lamina. If this clear space is maintained in the face of the thyroid lamina irregularity adjacent to the tumor, the lamina abnormality can be attributed to uneven calcification rather than tumor destruction. The posterior portion (lamina) of the cricoid cartilage (CC) is seen. The outer and inner cortex of the cartilage is calcified; an intervening marrow space has lower density. The vertical height of the lamina is 2 to 3 cm. There is incomplete calcification of the thyroid cartilage anteriorly. ICA, internal carotid artery; IJV, internal jugular vein; T, thyroid gland. C: Normal CT anatomy 5 mm below the free margin of the true vocal cord (TVC). The vocal cord appears thin because of abduction during scanning. There is incomplete bilateral paramedian calcification and thinning of the thyroid lamina (arrows). Notice the normal lack of tissue density between the airway and the anterior arch of the thyroid cartilage. CC, cricoid cartilage; CT, cricothyroid joint. (Million RR, Cassisi NJ. Larynx. In Million RR, Cassisi NJ, eds. Management of head and neck cancer: a multidisciplinary approach. Philadelphia: JB Lippincott, 1984:315–364.)
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
Carcinoma arising on the true vocal cords produces hoarseness at a very early stage. Sore throat, ear pain, pain localized to the thyroid cartilage, and airway obstruction are features of advanced lesions.
Hoarseness is not a prominent symptom of cancer of the supraglottis until the lesion becomes extensive. Pain on swallowing, usually mild, is the most frequent initial symptom, often described as a sore throat. Some patients report a sensation of a “lump in the throat.” Pain is referred to the ear by way of the vagus nerve and auricular nerve of Arnold. A mass in the neck may be the first sign of a supraglottic cancer. Late symptoms include weight loss, foul breath, dysphagia, and aspiration.
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
Physical Examination
Flexible fiberoptic endoscopes are used routinely to complement the laryngeal mirror examination. The mirror often provides the best view of the posterior pharyngeal wall. The flexible fiberoptic laryngoscope is inserted through the nose and is useful in more difficult cases.
Determination of vocal cord mobility frequently requires multiple examinations because the subtle distinctions between mobile, partially fixed, and fixed cords are often challenging, apparently changing from examination to examination. A cord that appeared mobile before direct laryngoscopy may exhibit impaired motion or even fixation after biopsy.
Ulceration of the infrahyoid epiglottis or fullness of the vallecula is an indirect sign of pre-epiglottic space invasion. Palpation of diffuse, firm fullness above the thyroid notch with widening of the space between the hyoid and the thyroid cartilages signifies invasion of the pre-epiglottic space. The pre-epiglottic fat space is a low-density area on the CT scan, and changes resulting from tumor invasion are easily seen.
Postcricoid extension may be suspected when the laryngeal click disappears on physical examination. Postcricoid tumor may cause the thyroid cartilage to protrude anteriorly, producing a fullness of the neck.
Invasion of the thyroid cartilage remains a difficult clinical diagnosis. Localized pain or tenderness to palpation or a small bulge over one ala of the thyroid cartilage is suggestive.
Radiographic Studies
CT scan with contrast enhancement is the method of choice for studying the larynx (Fig. 47.6).19 The CT scan should be performed before biopsy so that abnormalities that may be caused by the biopsy are not confused with tumor. CT is preferred to magnetic resonance (MR) imaging because the longer scanning time for MR results in motion artifact.20 CT slices 1 to 2 mm thick are obtained at 1- to 2-mm intervals through the larynx and at 3-mm intervals for the remainder of the study. Thinner sections (1 to 2 mm through the larynx) facilitate high-quality multiplanar reformations. The gantry is angled so that the scan slices are parallel to the plane of the true vocal cords. It is also necessary to obtain a CT scan of the entire neck to detect positive, nonpalpable lymph nodes. Positive retropharyngeal nodes may be present at diagnosis in patients with laryngeal cancer who have advanced neck disease.21 Retropharyngeal adenopathy is often not apparent on physical examination but is usually appreciated on CT scan.
Contrast enhancement helps to outline the blood vessels and thyroid gland. Tumor is often enhanced, probably because of reactive inflammatory changes. In addition to CT, MR may be obtained to define subtle exolaryngeal spread or early cartilage destruction. The value of MR for detecting early cartilage destruction is open to speculation. Sagittal MR may be useful in detecting early invasion of the base of the tongue.
Vocal Cord Carcinoma
Although the CT scan does not show minimal mucosal lesions and is generally not helpful for well-defined, easily visualized T1, or early T2 vocal cord carcinomas, it is almost always obtained. CT is excellent for determining subglottic extension and is often used in selected T1 and most T2 lesions for this reason alone. CT scanning is useful in the diagnosis of moderately advanced and advanced lesions; it is excellent for demonstrating extension outside the larynx into the soft tissues of the neck and has potential for determining thyroid or cricoid cartilage invasion, which tends to occur at the edges of the cartilage rather than on the faces. Early cartilage involvement is difficult to detect with axial scans, but it may be demonstrated by coronal or sagittal scanning techniques. If the low-density plane of the paraglottic space is intact, cartilage is probably not invaded by tumor.
Archer et al.22 correlated CT findings with the incidence of cartilage or bone invasion on whole-organ sections. For 12 of 14 patients with pathologic evidence of cartilage invasion, the average diameter of the tumor in two dimensions was >16 mm, and the lesion was located below the top of the arytenoid. Lesions in which the maximum diameter lay above the top of the arytenoid had a low incidence of cartilage invasion.22
Supraglottic Carcinoma
The CT scan provides an excellent means for viewing the pre-epiglottic and paraglottic fat spaces. Soft-tissue extension into the neck or base of the tongue can also be seen. The CT scan is also useful for determining extension to the subglottis.2
Diagnostic procedures for laryngeal cancer at the University of Florida are summarized in Table 47.1.23 A CT scan is usually performed for all patients; MR is obtained in a small subset of patients with questionable findings on CT. Positron emission tomography is not routinely obtained. Direct laryngoscopy and biopsy with frozen section are usually performed with the patient under general anesthesia. The ventricles, subglottis, apex of the pyriform sinus, and postcricoid area must be carefully examined because these areas are not consistently seen by indirect examinations. Fiberoptic telescopes (0 and 30 degrees) are introduced through the laryngoscope for inspection of these areas. A generous biopsy specimen is taken from the obvious lesion; additional biopsy specimens may be obtained from suspicious areas and from areas grossly involved. The mucosa of the margin of the cord may be stripped to provide adequate tissue if the lesion is distributed superficially along the cord and is not obviously a carcinoma.
TABLE 47.1 DIAGNOSTIC WORKUP FOR CARCINOMA OF THE LARYNX

TABLE 47.2 STAGING OF LARYNGEAL CANCER

 STAGING
STAGING
The 2010 American Joint Committee on Cancer (AJCC)24 staging system for laryngeal primary cancer is listed in Table 47.2. T2 glottic cancers are stratified into those with normal (T2A) and impaired (T2B) vocal cord mobility. For lesions arising in the supraglottis, the sites of origin include false cords, aryepiglottic folds, suprahyoid epiglottis, infrahyoid epiglottis, pharyngoepiglottic folds, and arytenoids. Only in the early T stages can one identify the specific site of origin with certainty. As the lesion enlarges, the site of origin is an educated guess based on the location of the greatest bulk of tumor. The major difference between the 1998 and 2010 staging systems is that a glottic cancer that invades the paraglottic space is upstaged to T3 in the latter system, even with mobile vocal cords, resulting in significant stage migration.25 In addition, T4 has been stratified into T4A and T4B, based on resectability.
 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
Nearly all malignant tumors of the larynx arise from the surface epithelium and therefore are squamous cell carcinoma or one of its variants.
Carcinoma in situ occurs frequently on the vocal cords. Differentiating among dysplasia, carcinoma in situ, squamous cell carcinoma with microinvasion, and true invasive carcinoma is a problem that the pathologist and the clinician frequently confront.
Most vocal cord carcinomas are well or moderately well differentiated. In a few cases, an apparent carcinoma and sarcoma occur together, but most of these are actually a spindle-cell carcinoma (i.e., squamous cell carcinoma with a spindle-cell stromal reaction).
Verrucous carcinoma occurs in 1% to 2% of patients with carcinoma of the vocal cord. The histologic diagnosis is difficult and must correlate with the gross appearance of the lesion.
Small-cell neuroendocrine carcinoma is rarely diagnosed in the supraglottic larynx, but it should be recognized because of its biologic potential for rapid growth, early dissemination, and responsiveness to chemotherapy.
Minor salivary gland tumors arise from the mucous glands in the supraglottic and subglottic larynx, but they are rare.26 Even rarer are paragangliomas, carcinoids, soft-tissue sarcomas, malignant lymphomas, and plasmacytomas. Benign chondromas and osteochondromas are reported, but their malignant counterparts are rare.
 PROGNOSTIC FACTORS
PROGNOSTIC FACTORS
The extent of the primary lesion and neck disease are the major determinants of prognosis. The likelihood of local control is determined primarily by T stage; there are conflicting data pertaining to a possible inverse relationship between N stage and local control. The likelihood of local-regional control is affected primarily by the overall AJCC stage, which accounts for both T stage and N stage. AJCC stage and N stage are the major determinants of cause-specific survival. In addition, within each N stage, patients with positive nodes in the low neck below the level of the thyroid notch tend to have a lower cause-specific survival rate than those with disease confined to the upper neck. In general, women tend to have a better prognosis than men.
 TREATMENT SELECTION AND TECHNIQUE: VOCAL CORD CARCINOMA
TREATMENT SELECTION AND TECHNIQUE: VOCAL CORD CARCINOMA
Selection of Treatment Modality
In treating vocal cord carcinoma, the goal is cure with the best functional result and the least risk of a serious complication. Patients may be considered to be in an early group if the chance of cure with larynx preservation is high, a moderately advanced group if the likelihood of local control is 60% to 70% but the chance of cure is still good, and an advanced group if the chance of cure is moderate and the likelihood of laryngeal preservation is relatively low. The early group may be treated initially by radiotherapy (RT) or, in selected cases, by partial laryngectomy. The moderately advanced group may be treated with either RT with laryngectomy reserved for relapse or by total laryngectomy with or without adjuvant postoperative RT. The obvious advantage of the former strategy, which we use at the University of Florida, is that there is a fairly good chance that the larynx will be preserved.27 Although some patients may be rehabilitated with a tracheoesophageal puncture after laryngectomy, only about 20% of patients use this device long term, and the majority use an electric larynx.28 The advanced group is treated with total laryngectomy and neck dissection with or without adjuvant RT or by RT and adjuvant chemotherapy.29 Data suggest that if patients whose tumors show a partial or complete response to two to three cycles of neoadjuvant chemotherapy are then given high-dose RT, the cure rates are comparable with those obtained with initial total laryngectomy.30 Another less expensive and less toxic method to select patients likely to be cured by RT alone is to calculate the primary tumor volume on pretreatment CT or MR. Data indicate that primary tumor volume is inversely related to the probability of local control after irradiation.31,32 Recent data indicate that whereas induction chemotherapy probably does not improve the likelihood of local-regional control and survival, concomitant chemotherapy and RT results in an improved possibility of cure compared with RT alone.33–35 There is a subset of patients with high-volume (>3.5 cc), unfavorable, advanced cancers who may be cured by chemoradiation but have a useless larynx and permanent tracheostomy and/or gastrostomy.31 These patients are best treated with a total laryngectomy, neck dissection, and postoperative RT.
Carcinoma in Situ
Lesions diagnosed as carcinoma in situ may sometimes be controlled by stripping the cord. However, it is difficult to exclude the possibility of microinvasion on these specimens. Recurrence is frequent, and the cord may become thickened and the voice hoarse with repeated stripping. Localized carcinoma in situ can also be excised using the CO2 laser.
Early RT for carcinoma in situ often means a better chance of preserving a good voice, especially since many patients with this diagnosis eventually receive this treatment.36
Many patients with a diagnosis of carcinoma in situ have obvious lesions that probably contain invasive carcinoma. We have often proceeded with RT rather than put the patient through a repeated biopsy procedure.
Early Vocal Cord Carcinoma
In most centers, RT is the initial treatment prescribed for T1 and T2 lesions, with surgery reserved for salvage after RT failure.17,37,38 Although hemilaryngectomy or cordectomy produces comparable cure rates for selected T1 and T2 vocal cord lesions, RT is generally preferred.37,39 Supracricoid laryngectomy, as reported by Laccourreye et al.,40 is a procedure designed to remove moderate-sized cancers involving the supraglottic and glottic larynx. The larynx may be removed with preservation of the cricoid and the arytenoid with its neurovascular innervation; the defect is closed by approximating the base of the tongue to the remaining larynx. The oncologic and functional results of this procedure in selected patients are reported to be excellent. Transoral laser excision also may provide high cure rates for select patients with small, well-defined lesions limited to the mid one-third of one true cord.41–45 A small subset of transoral laser surgeons successfully use this technique in moderately advanced cancers.37 The major advantage of RT compared with partial laryngectomy is better quality of the voice. Partial laryngectomy finds its major use as salvage surgery in suitable cases after RT failure. Even if the patient has a local recurrence after salvage partial laryngectomy, there is a third chance with total laryngectomy, which may still be successful.
Verrucous lesions have the reputation of being unresponsive to RT and, in some instances, converting into invasive, often anaplastic, metastasizing lesions. Partial laryngectomy is recommended for early verrucous carcinoma of the glottis, but RT is recommended if the alternative is total laryngectomy. We have observed typical verrucous lesions that have disappeared with RT and not recurred. O’Sullivan et al.46 also made this observation. In addition, a variety of tumors that recur after unsuccessful treatment (with surgery, RT, and/or chemotherapy) are more likely to exhibit more aggressive behavior.
Moderately Advanced Vocal Cord Cancer
Fixed-cord lesions (T3) may be subdivided into relatively favorable or unfavorable lesions. Patients with unfavorable lesions usually have extensive bilateral disease with a compromised airway and are considered to be in the advanced group. Patients with favorable T3 lesions have disease confined mostly to one side of the larynx, have a good airway, and are reliable for follow-up. Some degree of supraglottic and subglottic extension usually exists. The extent of disease and tumor volume, in particular, are related to the likelihood of control after RT.31
The patient with a favorable lesion is advised of the alternatives of RT with surgical salvage or immediate total laryngectomy. Recent data suggest that the likelihood of local-regional control is better after some altered fractionation schedules compared with conventional once-daily RT.35,47 Follow-up examinations are recommended every 4 to 6 weeks for the first year, every 6 to 8 weeks for the second year, every 3 months for the third year, every 6 months for the fourth and fifth years, and annually thereafter. The patient must understand that total laryngectomy may be recommended purely on clinical grounds without biopsy-proven recurrence and that the risk of laryngeal osteochondronecrosis is about 5%.
Evaluation of cord mobility after 50.4 Gy or at the end of RT has not been helpful in predicting local control.32 Some patients in whom the vocal cord remained fixed have had local tumor control of the disease for 2 years or longer after RT.
The major difficulty in using RT for the more advanced lesions is distinguishing radiation edema from local recurrence during follow-up examinations.48 Progressive laryngeal edema, persistent throat pain, or fixation of a previously mobile vocal cord frequently signifies recurrent disease in the larynx, although a few patients with these findings remain disease-free with long-term follow-up.
Extended hemilaryngectomy has been used by a few surgeons in the treatment of well-lateralized fixed-cord lesions. A permanent tracheostomy is usually required because a portion of the cricoid is resected, but a useful voice may be retained.49
Advanced Vocal Cord Carcinoma
Advanced lesions usually show extensive subglottic and supraglottic extension, bilateral glottic involvement, and invasion of the thyroid, cricoid, and/or arytenoid cartilages.9,22 The airway is compromised, necessitating a tracheostomy at the time of direct laryngoscopy in approximately 30% of patients. Clinically positive lymph nodes are found in about 25% to 30% of patients.
The mainstay of treatment is total laryngectomy, with or without adjuvant RT. The most frequent sites of local failure after total laryngectomy are the tracheal stoma, the base of the tongue, the neck lymph nodes, and/or or soft tissues of the neck. If the neck is clinically negative before surgery and if postoperative RT is planned, neck dissection may be withheld, and RT may be used to treat both sides of the neck. However, in practice, most surgeons prefer to perform elective bilateral selective (levels II to IV) neck dissections in conjunction with a total laryngectomy for T3 N0 or T4 N0 laryngeal cancer, even if postoperative RT is planned. If the lymph nodes are clinically positive, a therapeutic neck dissection is performed at the time of laryngectomy.
The indications for postoperative RT include close or positive margins, significant subglottic extension (1 cm or more), cartilage invasion, perineural invasion, endothelial-lined space invasion, extension of the primary tumor into the soft tissues of the neck, multiple positive neck nodes, extracapsular extension, and control of subclinical disease in the opposite neck.50,51 Preoperative RT is indicated for patients who have fixed neck nodes, have had an emergency tracheotomy through tumor, or have direct extension of tumor involving the skin.
Definitive RT is prescribed for the patient who refuses total laryngectomy or is medically unsuitable for major surgery.
As previously stated, there is evidence that two to three cycles of induction chemotherapy followed by RT in patients obtaining at least a partial response may provide a moderate likelihood of larynx preservation without compromising cure.30 Data suggest that concomitant chemotherapy and RT is more efficacious than RT alone or induction chemotherapy followed by RT.33,34 The optimal combination of concomitant chemotherapy and irradiation is unclear.35
A randomized intergroup trial (RTOG 91-11) compared three treatment arms: arm A, three cycles of induction cisplatin and fluorouracil followed by RT in complete and partial responders; arm B, RT and concomitant cisplatin (100 mg/m2 on days 1, 22, and 43 of RT); and arm C, once-daily RT (70 Gy in 35 fractions over 7 weeks) alone.33 Five hundred forty-seven patients were randomized and followed for a median of 3.8 years; 518 patients were evaluable. The rates of larynx preservation were as follows: arm A, 72%; arm B, 84%; and arm C, 67%. The rates of larynx presentation were significantly improved for arm B; there was no significant difference between arms A and C. The 5-year survival rates were similar for the three treatment groups: arm A, 55%; arm B, 54%; and arm C, 56%. The likelihood of developing distant metastases was lower for the two groups of patients that received adjuvant chemotherapy.
Surgical Treatment
Cordectomy is an excision of the vocal cord and may be performed by the transoral approach usually with a laser or externally by a thyrotomy. Its use is usually confined to small lesions of the middle one-third of the cord. After cordectomy, a pseudocord is formed, and the patient has a useful, if somewhat harsh, voice.
Vertical partial laryngectomy (i.e., hemilaryngectomy) allows removal of limited cord lesions with preservation of voice. One entire cord with as much as one-third of the opposite cord with the adjacent thyroid cartilage is the maximum cordal involvement suitable for surgery in men; women have a smaller larynx, and usually only one vocal cord may be removed without compromising the airway. Partial fixation of one cord is not a contraindication to hemilaryngectomy; a few surgeons performed a hemilaryngectomy for selected fixed-cord lesions. The maximum subglottic extension suitable for hemilaryngectomy is 8 to 9 mm anteriorly and 5 mm posteriorly; this limit is necessary to preserve the integrity of the cricoid. Tumor extension to the epiglottis, false cord, or both arytenoids is a contraindication to hemilaryngectomy.
Supracricoid partial laryngectomy is used for selected T2 and T3 glottic carcinomas and entails removal of both true and false cords as well as the entire thyroid cartilage. The cricoid is sutured to the epiglottis and hyoid (cricohyoidoepiglottopexy).
Total laryngectomy with or without neck dissection is the operation of choice for advanced lesions and as a salvage procedure for RT failures in lesions that are not suited for conservation surgery. The entire larynx is removed, and the pharynx is reconstructed. A permanent tracheostomy is required. Speech may be reconstituted with a prosthesis or with an electrolarynx. One hundred four (63%) of 166 patients entered into the surgery and postoperative irradiation arm of the Veterans Affairs Laryngeal Cancer Study Group randomized trial were evaluable for communication status at 2 years after treatment.52 Ninety-six patients had undergone a total laryngectomy and communicated as follows: tracheoesophageal, 27 (28%); esophageal, 5 (5%); artificial larynx, 47 (50%); nonvocal, 7 (7%); and no data, 10 (10%).52 One hundred seventy-three patients underwent total laryngectomy and postoperative RT at the University of Florida, and 69 patients were evaluable for 5 years or longer.28 Voice rehabilitation was accomplished as follows: tracheoesophageal, 19%; artificial larynx, 57%; esophageal, 3%; nonvocal, 14%; and no data, 7%.
Radiation Therapy Technique
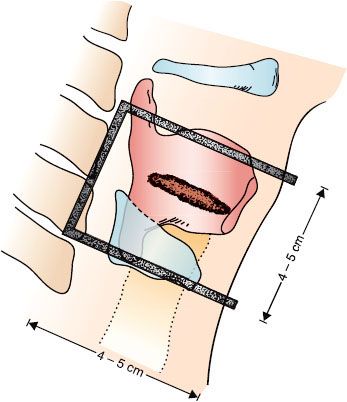
RT for T1 or T2 vocal cord cancer is delivered by small portals covering only the primary lesion.38 The cervical lymph node chain is not electively treated. For T1 lesions, RT portals extend from the thyroid notch superiorly to the inferior border of the cricoid and fall off anteriorly. The posterior border depends on the posterior extension of the tumor.20 For T2 tumors, the field is extended depending on the anatomic distribution of the tumor. The field size ranges from 4 × 4 cm to 5 × 5 cm (plus an additional 1.0 cm of “flash” anteriorly) and is occasionally 6 × 6 cm for a large T2 lesion. Portals larger than this increase the risk of edema without improving the cure rate.
A commonly used dose-fractionation schedule at many institutions is 66 Gy for T1 lesions and 70 Gy for T2 cancers given in 2-Gy fractions. Evidence suggests that increasing the dose per fraction may improve the likelihood of local control.53–57 Ample data suggest that 1.8 Gy once daily results in significantly lower local control rates compared with 2.0 Gy once daily.54 Yamakazi et al.58 reported a prospective trial in which patients with T1 N0 squamous cell carcinoma of the glottic larynx were randomized to definitive RT at 2.0 Gy per fraction or 2.25 Gy per fraction. The 5-year local control rates were 77% after 2.0 Gy per fraction and 92% after Gy per fraction (p = .004); there was no difference in either acute or late toxicity. Patients with T1 or T2 vocal cord cancer treated with once-a-day fractionation at the University of Florida are irradiated with 2.25-Gy fractions; the dose-fractionation schemes used are as follows: Tis–T2 A, 63.0 Gy in 28 fractions, and T2B, 65.25 Gy in 29 fractions.
At the University of Florida, patients are treated in the supine position; the field borders for a patient with a T1 N0 cancer are depicted in Figure 47.7.20 The field is checked by the physician at the treatment machine according to palpable anatomic landmarks. This allows the treatment volume to be kept at a minimum and reduces the risk of geographic miss. A three-field technique, using 4- or 6-MV x-rays, is used to deliver approximately 95% of the dose through opposed lateral wedged fields weighted to the side of the lesion; the remaining dose is delivered by an anterior field shifted 0.5 cm toward the side of the lesion (Fig. 47.8).20 The tumor dose is usually specified at the 95% normalized isodose line.
RT of T3 and T4 lesions requires larger portals, which include the levels II and III lymph nodes (Fig. 47.9).59,60 The level IV lymph nodes are included in a separate low-neck portal. Patients treated at the University of Florida are irradiated in a continuous course twice daily at 1.2 Gy per fraction to a total dose of 74.4 Gy. The portals are reduced after 45.6 Gy in 38 fractions; the reduced portals cover only the primary lesion.
Intensity-modulated RT (IMRT) is used if there is a clear advantage associated with this technique. Disadvantages associated with IMRT include increased dose inhomogeneity, increased total body dose, and increased labor and expense.61 The most common indications for IMRT for laryngeal cancers would be the occasional patients with a node-positive T3–T4 cancer, where the retropharyngeal nodes would be electively irradiated and the dose to the contralateral parotid gland reduced, and/or a difficult low match between the lateral fields used to treat the primary site and upper neck and the anterior low neck field in a patient with a short neck and large shoulders. In the latter instance, IMRT could be used to encompass the entire target volume and avoid the problem of field junctioning entirely. IMRT is especially useful for patients with extensive subglottic invasion, where achieving an adequate inferior margin with conventional lateral portals may not be possible.
FIGURE 47.7. Treatment portal for early glottic carcinoma. The top border is adjusted according to the lesion. The middle of the thyroid notch is the landmark for very early lesions, and the top of the notch is the marker for larger lesions or those with minimal supraglottic extension. The posterior border is 1 cm posterior to the back edge of the thyroid cartilage if the lesion is confined to the anterior two-thirds of the vocal cord; if the posterior one-third of the vocal cord is involved, the posterior border is placed 1.0 to 1.5 cm behind the cartilage. The inferior border is placed at the bottom of the cricoid cartilage if there is no subglottic extension. (From Million RR, Cassisi NJ, Mancuso AA. Larynx. In: Million RR, Cassisi NJ, eds. Management of head and neck cancer: a multidisciplinary approach, 2nd ed. Philadelphia, JB Lippincott, 1994;431–497.)