Chapter 11 Laboratory methods used in the investigation of the haemolytic anaemias
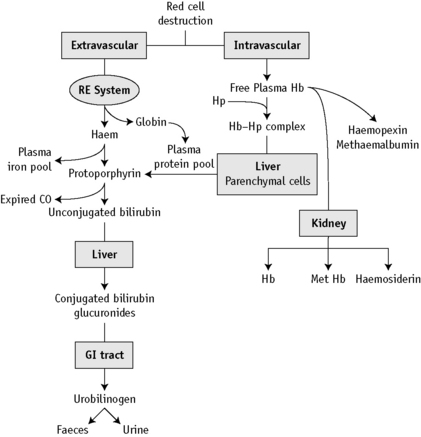
At the end of a normal lifespan, red cells are destroyed within the reticuloendothelial system in the spleen, liver and bone marrow. In some haemolytic anaemias, the haemolysis occurs predominantly in the reticuloendothelial system (extravascular) and the plasma haemoglobin concentration (Hb) is barely increased. In other disorders, a major degree of haemolysis takes place within the bloodstream (intravascular haemolysis), the plasma Hb increases substantially and in some cases, the amount of Hb so liberated may be sufficient to lead to Hb being excreted in the urine (haemoglobinuria). However, there is often a combination of both mechanisms. The two pathways by which Hb derived from effete red cells is metabolized are illustrated in Figure 11.1.
Investigation of haemolytic anaemia
Is There Evidence of Increased Haemolysis?
What is the Precise Diagnosis?
Plasma haemoglobin
Methods for estimation of plasma Hb are based on (1) peroxidase reaction and (2) direct measurement of Hb by spectrometry. In the peroxidase method, the catalytic action of haem-containing proteins brings about the oxidation of tetramethylbenzidine* by hydrogen peroxide to give a green colour, which changes to blue and finally to reddish violet. The intensity of reaction may be compared using a spectrometer with that produced by solutions of known Hb. Hi and Hb are measured together.
A pink tinge to the plasma is detectable by eye when the Hb is higher than 200 mg/l. When the plasma Hb is >50 mg/l, it can be measured as haemiglobincyanide (HiCN) or oxyhaemoglobin by a spectrometer at 540 nm1 (p. 26). Lower concentrations can also be measured reliably provided that the spectrometer plots of concentration/absorbance give a linear slope passing through the origin. This facility is provided by the Low Hb HemoCue (Hemocue Ltd, Dronfield, Derbyshire, UK), which can reliably measure plasma Hb at or higher than 100 mg/l.2
Spectrophotometric Method
Normal Range
The normal range is 10–40 mg/l.
Significance of increased plasma haemoglobin
Hb liberated from the intravascular or extravascular breakdown of red cells interacts with the plasma haptoglobins to form an Hb–haptoglobin complex,4 which, because of its size, does not undergo glomerular filtration, but it is removed from the circulation by and is degraded in, reticuloendothelial cells. Hb in excess of the capacity of the haptoglobins to bind it passes into the glomerular filtrate; it is then partly excreted in the urine in an uncomplexed form, resulting in haemoglobinuria, and partly reabsorbed by the proximal glomerular tubules where it is broken down into haem, iron and globin. The iron is retained in the cells and eventually excreted in the urine (as haemosiderin). The haem and globin are reabsorbed into the plasma.
The haem complexes with albumin forming methaemalbumin and with haemopexin (p. 235); the globin competes with Hb to form a complex with haptoglobin. In effect, the plasma Hb level is further increased in haemolytic anaemias when haemolysis is sufficiently severe for the available haptoglobin to be fully bound. The highest levels are found when haemolysis takes place predominantly in the bloodstream (intravascular haemolysis). Thus, marked haemoglobinaemia, with or without haemoglobinuria, may be found in PNH, paroxysmal cold haemoglobinuria, cold-haemagglutinin syndromes, blackwater fever, march haemoglobinuria and other mechanical haemolytic anaemias (e.g. that after cardiac surgery). In warm-type autoimmune haemolytic anaemias, sickle cell anaemia and severe β thalassaemia, the plasma Hb level may be slightly or moderately increased, but in hereditary spherocytosis, in which haemolysis occurs predominantly in the spleen, the levels are normal or only very slightly increased.
Haem within the proximal tubular epithelium undergoes further degradation to bilirubin with liberation of iron, some of which is retained intracellularly incorporated into ferritin and haemosiderin. When haemolysis is severe, the excess of Hb that occurs in the glomerular filtrate will lead to an accumulation of intracellular haemosiderin in the glomerular tubular cells; when these cells slough, haemosiderin will appear in the urine (p. 236).
Increased levels may occur as a result of violent exercise, as well as occurring in runners and joggers as a result of mechanical trauma caused by continuous impact of the soles of the feet with hard ground.4
Serum haptoglobin
Direct measurement of haptoglobin is also possible by turbidimetry or nephelometry and by radial immunodiffusion.5 The methods described below are cellulose acetate electrophoresis and radial immunodiffusion.
Electrophoresis Method6,7
Reagents
Buffer (pH 7.0, ionic strength 0.05)
Na2HPO4.H2O 7.1 g/l, 2 volumes; NaH2PO4.H2O 6.9 g/l, 1 volume. Store at 4°C.
Method
Impregnate cellulose acetate membrane filter strips (12 × 2.5 cm) in buffer solution and blot to remove all obvious surface fluid. Apply 0.75 ml samples of the serum–haemolysate mixtures across the strips as thin transverse lines. As controls, include strips with serum alone and Hb lysate alone. Electrophorese at 0.5 mA/cm width. Good separation patterns about 5–7 cm in length should be obtained in 30 min (see Fig. 11.2).