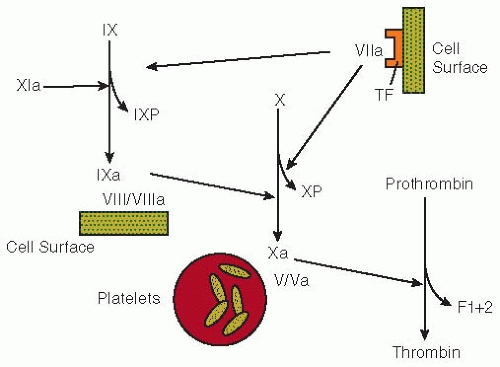
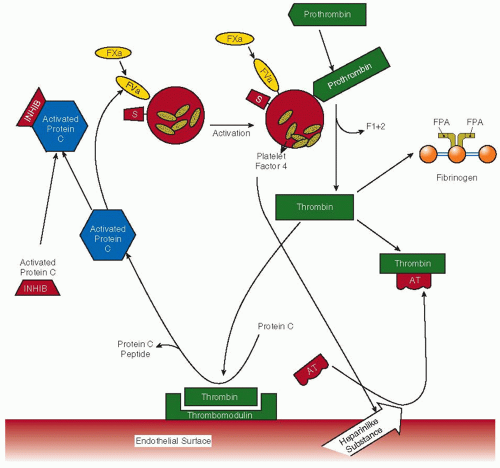
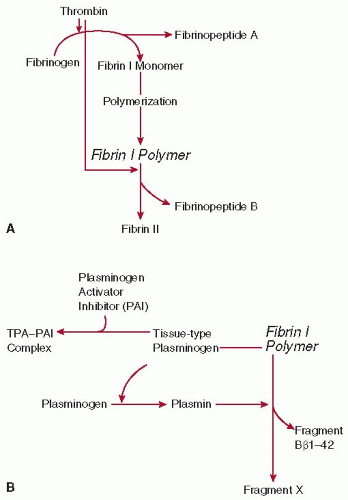
Influence of Physiologic Factors and Organ Dysfunction
A number of variables can result in overlap of assay results between individuals with a pathologic condition or altered physiologic status and health. Although values further from the mean of the normal distribution are more likely to be abnormal, appropriate interpretation requires an appreciation of the factors that can influence measurements.
The normal aging process alters coagulation activation in a predictable fashion.
18,98,99 With advancing age from 45 to 70 years, an increasing number of patients who are otherwise healthy exhibit elevated levels of F1+2. This reflects increased thrombin generation because clearance of radiolabeled F1+2 is unchanged.
98 The levels of the factor IX and X activation peptides also increase with advancing age.
10 Significant, but somewhat less striking, positive correlations have been observed between increasing age and the levels of fibrinopeptide A and protein C activation peptide.
98 In a study of 25 healthy Italian centenarians, coagulation and fibrinolysis measurements were compared with two control groups of healthy adults ranging from 18 to 50 years and 51 to 69 years.
99 Older controls generally had slightly higher values of several measurements than younger controls. Centenarians had striking signs of heightened coagulation enzyme activity as assessed by plasma measurements of factor VIIa (
P < 0.01 compared with either control group) or the activation peptides of factor IX, factor X, and prothrombin and TAT complexes (
P < 0.001 for all). Heightened coagulation enzyme activity was accompanied by enhanced formation of fibrin (high fibrinopeptide A and D-dimer,
P < 0.001) and secondary fibrinolysis (plasmin-antiplasmin complex,
P < 0.001).
It has been reported that strenuous exercise in the form of long-distance running leads to increased TAT complex levels without elevations in the levels of fibrinopeptide A.
100 Cigarette smoking has been associated with elevations in activation markers of blood coagulation.
101Certain medications that are not known to have a direct effect on blood coagulation can alter the activity of the coagulation mechanism. Caine et al.
102 showed that administration of conjugated equine estrogen daily to menopausal women for 3 months increased the levels of F1+2 in a dose-dependent manner. The activity of fibrinopeptide A also increased, and levels of protein S and antithrombin were decreased as compared to placebo treatment. In women who had undergone oophorectomy, Kroon et al.
103 confirmed that 0.625 mg of conjugated equine estrogen, as well as 0.05 transdermal 17β-estradiol daily for 6 weeks increased levels of F1+2. Scarabin et al.
104 found that 2 mg of estrogen valerate daily with cyclic progesterone increased F1+2 levels and decreased antithrombin activity, whereas transdermal estrogen (2.5 mg 17β-estradiol daily with cyclic progesterone) did not. The treatment of patients with coronary heart disease with gemfibrozil, a drug used to lower serum cholesterol and triglyceride concentrations, reduces F1+2 levels by approximately 25%.
105In healthy volunteers, postprandial elevations in the levels of factors VIIa and IX activation peptide have been demonstrated, but no changes were observed in the levels of factor XIIa or indices of thrombin generation.
106,107,108 The roles of factors XII, XI, and IX in factor VII activation were evaluated by investigating patients with isolated deficiencies of these factors after a fatty meal.
106,108 Factor VIIa levels increased postprandially in patients with factor XII deficiency but did not change in those with factor IX deficiency. In patients with factor XI deficiency, Miller et al.
106 found that factor VIIa levels rose postprandially, whereas Silveira et al.
108 did not observe significant alterations. From these studies, it can be concluded that factor IX plays a critical role in the events linking postprandial lipemia to factor VII activation, but factor XII does not. This implies that factor XII is not involved in the activation of factor VII by lipolysis of triglyceride-rich lipoproteins.
Dysfunction in normal physiologic clearance mechanisms can also result in substantial elevations in the levels of activation peptides. For F1+2, this has been demonstrated in patients with chronic renal failure on dialysis.
109,110 Caution is required, therefore, in interpreting an elevated level of a marker as evidence of heightened coagulation or fibrinolytic activity in disorders associated with renal (e.g., thrombotic thrombocytopenic purpura, systemic lupus erythematosus, nephrotic syndrome, renal transplant rejection) or hepatic dysfunction. In a prospective epidemiologic study of healthy middle-aged males, the Second Northwick Park Heart Study (NPHSII) measured the levels of F1+2, fibrinopeptide A, factor IX activation peptide, factor X activation peptide, factor VIIa, and factor XIIa. The factor IX activation peptide was the only coagulation activation marker found to correlate with creatinine.
111To determine the influence of inflammation on levels of activated markers of coagulation, correlation analysis was done between C-reactive protein (CRP) and factor XIIa, factor VIIa, factor IX activation peptide, factor X activation peptide, F1+2, and FPA in healthy individuals within NPHSII.
111 Only weak significant correlations (Pearson correlation 0.15,
n > 1,135) were observed for each analyte with CRP, suggesting that inflammation does not contribute a great deal to levels of coagulation activation markers. The Pearson correlation between CRP and fibrinogen in the same individuals was 0.45, suggesting a large influence of inflammation upon fibrinogen. Stronger correlations were, however, observed between the coagulation activation markers downstream from FVIIa-tissue factor. Factor IX activation peptide and factor X activation peptide were highly correlated (0.47,
n = 1,335), while the correlation coefficients between factor X activation peptide and prothrombin F1+2 or factor VIIa were 0.23 and 0.21, respectively.