L-Asparaginase
Most anticancer drugs are small synthetic molecules designed to inhibit enzymes or to interact with DNA. Newer drugs may also be proteins, such as monoclonal antibodies or cytokines (interferon, IL-2) that interact with cell surface receptors. L-asparaginase (L-ASP) is unique as a bacterial enzyme that hydrolyzes an essential amino acid, L-asparagine, and through that action, kills tumor cells. Its efficacy is based on the observation that some lymphoid malignancies are unable to synthesize asparagine and must derive it from the blood stream. Enzyme purified from Escherichia coli (1) is now an essential component of the regimen for remission induction and consolidation for childhood acute lymphocytic leukemia (ALL). While enzymatic activity is highly specific for asparagine hydrolysis, asparagine depletion not only kills tumor cells but also leads to a broad range of toxicities resulting from inhibition of the synthesis of clotting factors, insulin, and other essential proteins.
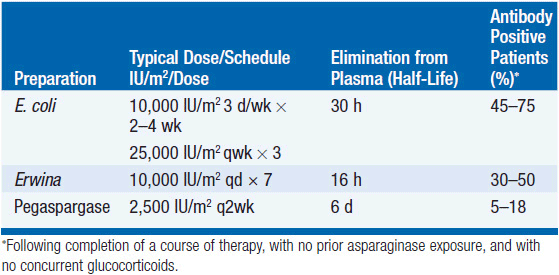
L-ASP is a 144,000 tetrameric protein that catalyzes the deamination of the circulating blood pool of L-asparagine. Enzyme from E. coli and an alternative protein from Erwinia chrysanthemi are highly specific for L-asparagine but retain minor cleaving activity against glutamine. The two enzymes lack immunologic cross-reactivity; therefore, the Erwinia enzyme may be used with relative safety in patients hypersensitive to E. coli enzyme, but because of its short plasma half-life of 16 h, must be administered in higher doses to produce prolonged asparagine depletion. A third form of L-ASP, the E. coli enzyme conjugated to polyethylene glycol (pegaspargase—PEG L-ASP), has a much long plasma t1/2 of 6 days, is less immunogenic than native L-ASP, and is particularly useful in patients hypersensitive to the unconjugated enzymes, 70% of whom will not react to the pegylated enzyme (2). PEG L-ASP is as effective as native E. coli enzyme in first-line use. A recombinant E. coli L-ASP, which is free of the immunogenic oligomers of the native bacterial enzyme preparation, is undergoing clinical evaluation and may prove to be a superior product (2).
CLINICAL PHARMACOLOGY
A comparison of the primary features of the three drugs is shown in Table 8-1. No single dosing schedule of the various L-ASP preparations has been established. The native L-ASP drug is primarily administered by intramuscular injection, a route associated with a lesser risk of anaphylaxis, although PEG L-ASP appears safe by the intravenous route (3). Higher doses are associated with more complete and prolonged asparagine depletion, but cause a higher incidence of side effects, particularly thrombotic events, and more frequent hypersensitivity responses. In general, most regimens strive to maintain a trough level of enzyme activity in plasma of 0.1 units/ml for the duration of therapy (for 7–21 days). This level is associated with total asparagine depletion in plasma but not in the cerebrospinal fluid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree