Chapter 54 Kidney and Ureteral Carcinoma
Renal cell carcinoma, historically referred to as hypernephroma or Grawitz’s tumor, was first reported by Konig in 1826. In 1883, Grawitz3 noted that the fatty content of RCC was similar to that of adrenal cells and postulated that these tumors arose from adrenal rests within the kidney. In 1894, Birch-Hirschfel used the term hypernephroid to describe these tumors.
Primary cancers of the renal pelvis and ureter are uncommon. About 5% of urothelial carcinomas occur in the kidneys and ureters.4 Radical nephroureterectomy, with removal of the bladder cuff, is the main means of treatment. In recent years, laparoscopic and robot-assisted surgeries have become available as alternatives to an open approach. The use of more conservative surgery should be limited to well-selected patients who have low-grade and low-stage disease, small unifocal lesions, solitary kidney, or significant medical comorbidity. Patients with high-grade tumors or advanced-stage disease are at significant risk of local as well as distant relapse. Adjuvant chemotherapy and/or radiation therapy may improve the treatment outcome in patients with locally advanced disease.
Etiology and Epidemiology
Kidney
Epidemiology
Kidney cancer comprises approximately 3% of new cancer cases and 3% of cancer deaths in the United States each year.1,5 In 2010 there were approximately 58,240 new cases of kidney and renal pelvis cancer diagnosed in the United States and approximately 13,040 deaths attributed to kidney and renal pelvis cancer. Over 90% of kidney cancers among adults are classified as RCC.5
RCC is typically diagnosed in the seventh decade of life, with a median age at diagnosis of 65 years; however, it has been observed in children as young as 6 months old. According to data from the Surveillance, Epidemiology and End Results (SEER) program, the overall incidence of RCC in the United States has increased at a rate of 2% to 3% per year since the early 1970s.6,7 Although the greatest increase in RCC incidence has been among localized tumors, early detection of subclinical tumors cannot fully explain the rising incidence of RCC. There is also an upward trend for more advanced tumors as well as an overall increase in mortality rates.6,8–10
There is a strong gender preponderance, with incidence rates in men approximately twice that of women.1 Men account for roughly two thirds of RCC diagnoses and deaths in the United States.11 There is also a notable variability in RCC incidence across racial and ethnic groups. The highest rates are reported for whites and African Americans, whereas the lowest rates are reported for Asian Americans. Between 1975 and 1998, incidence rates among African Americans increased by 4.5% compared with only 2.9% in whites. Globally, the incidence rate of RCC is the highest in North America and Scandinavia and the lowest is in Asia and South America.12
Etiology
Cigarette smoking and obesity are two well-established risk factors for RCC, and each may increase the risk of RCC roughly twofold. These factors may account for 40% of all RCCs diagnosed in the United States.13–20 Although hypertension is often included as a risk factor, this association is considered less certain, given its correlation with both smoking and obesity.13,16–18 Some reports suggest that hypertension may increase the risk of RCC approximately twofold.21,22 There may be an inverse association (i.e., protective effect) of RCC development with moderate alcohol consumption and physical activity, as well as a positive association with a history of urinary tract infections.23–30 There are few consistent associations of RCC and dietary factors.18,31 Consumption of vegetables may be associated with reduced risk, whereas red meat consumption may slightly increase the risk of RCC32–34; however, a recent meta-analysis did not support an independent association of red meat consumption and RCC.35 Finally, occupational exposures to asbestos, gasoline, lead, and cadmium, use of Thorotrast, and treatment with phenacetin, as well as a history of end-stage renal disease or acquired cystic kidney disease, have been associated with increased risk of RCC.36–41
A positive family history has been reported to increase the risk of RCC as much as twofold.42 Although genes that are associated with inherited forms of RCC have been identified, there are currently very few published data regarding the role of germline genetic susceptibility and sporadic RCC.17,43,44 Small case-control studies have demonstrated that individuals carrying polymorphisms in specific genes (particularly phase I enzymes) may be at increased risk of RCC.45–48 The results of these studies have yet to be validated. Large genome-wide association studies of RCC risk are not available at this time.
Renal Pelvis and Ureter
Epidemiology
Approximately 5% of urothelial neoplasms occur in the kidneys and ureters.4 Data from the SEER program suggest that the age-adjusted annual incidence of renal pelvic and ureteral cancers is 0.73/100,000 person-years.49 Similar to RCC, there is a strong male predominance. The incidence increases with age, with the peak age at diagnosis in the sixth and seventh decades of life. Primary tumors of the ureter occur only half as frequently as those of the renal pelvis. The incidence of UUT urothelial cancers has been increasing.49,50 Disease-specific annual mortality is greater in black than in white individuals and in women than in men.
Etiology
Risk factors and environmental agents that cause bladder cancer appear to play similar roles in the development of upper tract urothelial cancers.51–55 Patients with carcinomas of the renal pelvis have more than a 30% to 80% risk of developing metachronous or synchronous bladder cancer. The risk of developing UUT malignancy after treatment of superficial bladder cancer is up to 9.8%.54 The median time to the development of secondary UUT cancer after bladder cancer diagnosis is 33 months. Contralateral metachronous UUT cancer is relatively uncommon, occurring in 3% to 6% of patients.53,56
Cigarette smokers have a 2.6- to 7.2-fold increased risk of UUT cancer.57–59 Chronic use of analgesics that contain phenacetin has been implicated as a risk factor.59–61 Capillarosclerosis is a pathognomonic change seen in patients with long-standing abuse of compound analgesics.62 Ingestion of Chinese herbs containing Aristolochia fangchi, which is used for weight reduction, results in a nephropathy characterized by progressive renal fibrosis and increased risk of urothelial cancer.63,64 There is a 57- to 100-fold increase in the incidence of UUT cancer in Balkan countries affected by an endemic nephropathy.65,66 The renal pathology is an interstitial nephritis. Patients with tumors from the endemic region present with higher-grade disease and more solid growth pattern compared with those from nonendemic regions.67 The cause of the endemic nephropathy is postulated to be high concentrations of radon and minerals in the drinking water in the area. Occupational exposure to organic chemicals is associated with a higher risk of UUT cancers for workers in the chemical, petrochemical, or plastic industries.57 Patients with a history of kidney or ureteral stones have a 2.5-fold increase in risk of renal and ureteral cancers, suggesting that chronic irritation and infection may play a role.68 There are reports of kindred with familial urothelial cancers of the UUT.69–71
Prevention and Early Detection
Kidney
Cessation of cigarette smoking is currently the most effective method of preventing RCC. A large case-control study in Iowa reported that the risk of RCC development decreases steadily from the point of smoking cessation.72 After 15 years of cessation the risk of developing RCC returns to that of nonsmokers. It is unclear whether reduction in weight would decrease the risk for RCC. Some data suggest that daily moderate alcohol consumption and physical activity reduce the risk of RCC; however, more investigations are needed to confirm their benefits.24,25
Early diagnosis of RCC is challenging. Twenty-five percent to 40% of patients are asymptomatic at the time of diagnosis.73 Over the past 3 decades the percentage of patients presenting with the classic triad of flank pain, hematuria, and a palpable mass has dropped to less than 10%. The increased use of imaging modalities including CT, MRI, and ultrasonography helps to detect disease at earlier stages, which may translate to greater probability of resectability.74
Renal Pelvis and Ureter
Lifestyle modification targeting known risk factors of UUT cancers may help to prevent these diseases. Smoking cessation is extremely important, with the reduction in risk correlating with the time of quitting cigarette smoking.58 Diet with frequent intake of both green and yellow vegetables may also reduce the risk of urothelial cancers.75 However, there is no known intervention that can prevent future development of metachronous lesions of the urothelial tract after a primary tumor has been diagnosed.
The use of urine cytology as a screening test is not clearly defined, even in populations at increased risk of developing these diseases (e.g., heavy smokers, workers in petrochemical industries). For patients with a prior history of bladder cancer the use of screening intravenous pyelography as a routine follow-up examination is controversial.76
Pathology and Pathways Of Spread
Kidney
Pathology
In 1997, an international consensus conference on RCC sponsored by the Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) outlined recommendations for the classification of RCC.77 The classification system originally proposed at the Heidelberg conference in 1996 was adopted.78 RCC is categorized as clear cell, papillary, chromophobe, or collecting duct subtypes. RCC that does not fall into one of these four groups is classified as “renal cell carcinoma, not otherwise specified.” Granular cell RCC was excluded from the classification because it encompassed oncocytoma (benign kidney tumor) as well as chromophobe and clear cell RCC and was not specific to a single subtype. This classification system recognizes that RCC consists of several histologic subtypes with distinct morphologic and genetic characteristics. Previous reports have demonstrated significant differences in patient outcome based on histologic subtypes.79–81 Patients with clear cell RCC have a worse prognosis compared with patients with papillary and chromophobe RCC. There is no statistically significant difference in outcome between patients with papillary RCC and those with chromophobe RCC.82
Furthermore, features predictive of a poorer prognosis, including histologic tumor necrosis and sarcomatoid differentiation, have been shown to differ by subtype.80–83 Histologic tumor necrosis is noted in 20% to 45% of RCC patients, whereas sarcomatoid differentiation occurs in 5% to 15% of patients.80–82
Fuhrman and colleagues84 developed a nuclear grading system based on the size and appearance of nuclei and nucleoli. The Fuhrman nuclear grade is predictive of outcome in patients with RCC.
Algorithms have been developed to predict outcomes for patients with RCC. Using retrospective data from 1801 patients with clear cell RCC, Frank and associates85 from the Mayo Clinic developed a predictive model using an SSIGN scoring system that incorporated stage, tumor size, nuclear grade, and histologic tumor necrosis. Ten-year cancer-specific survival can be estimated based on a patient’s SSIGN score. Kattan and coauthors86 developed a nomogram to predict 5-year probability of treatment failure using presenting symptoms, histology, tumor size, and stage. Zisman and colleagues87 developed a clinical outcome algorithm based on 814 patients treated at the University of California, Los Angeles, using stage, Fuhrman grade, and Eastern Cooperative Oncology Group (ECOG) performance status. This algorithm has been validated by two international multicenter studies.88,89
Pathways of Spread
RCC may spread by local extension through the renal capsule into the perinephric fat or the adrenal gland or by direct extension through the renal vein to the inferior vena cava (occasionally reaching the right atrium). Regional lymphatic drainage includes renal hilar, paracaval, aortic, and retroperitoneal lymph nodes. RCC may also spread by blood-borne metastases to the lung, soft tissue, bone, and brain or by retrograde venous drainage to the ovary or testis.90
Renal Pelvis and Ureter
Pathology
Transitional cell carcinoma (TCC) accounts for the majority of renal pelvis and ureter neoplasms.91 Squamous cell carcinoma (SCC) accounts for about 4% of all cases.92 Although patients with SCC tend to present with more advanced-stage diseases and worse prognosis, stage for stage, there is no significant difference in prognosis between TCC and SCC. Other cell types such as adenocarcinoma and sarcoma are very rare.
Both stage and tumor grade are important prognostic factors for survival.93–98 Lymph node metastasis and lymphovascular space invasion are associated with stage and predict for worse outcome.99,100
Gross tumor architecture (sessile vs. papillary growth pattern) is an independent predictor of tumor relapse and cancer-specific mortality.101 Sessile growth pattern is associated with higher tumor grade, more advanced stage, and nodal metastasis. A history of prior bladder cancer has also been associated with worse disease-specific survival (DSS).102
Pathway of Spread
Involvement of multiple areas of the urothelial epithelium by TCC, either synchronously or metachronously, is common in patients with renal pelvis and ureteral cancers. As the tumor progresses, it invades the surrounding tissues with direct extension through the ureteral wall into periureteral tissues or to adjacent renal parenchyma as well as extrarenal tissues. Lymph node metastasis is the most common site of metastasis in UUT cancers. In a multi-institutional series of 1363 patients,103 lymph node metastasis was shown to increase with advancing pathologic stage: less than 1% for T0/Ta/Tis, 2% for T1, 8% for T2, 17% for T3, and 46% for T4.
Kondo and coauthors104 outline the distribution of nodal metastatic sites. For tumors of the right renal pelvis, the primary metastatic sites are the right renal hilar, paracaval, and retrocaval nodes. Tumors of the upper two thirds of the right ureter primarily metastasize to the retrocaval and interaortocaval nodes. Tumors of the left renal pelvis metastasize to the left renal hilar and para-aortic nodes. Tumors of the upper two thirds of the left ureter primarily metastasize to the para-aortic nodes. Tumors of the lower ureter primarily metastasize inferiorly to the aortic bifurcation. The finding of lymph vessel invasion in the tumor is predictive of regional lymph node metastasis.105 Nodal metastasis is a predictor for distant metastasis and is associated with worse survival.99,103
Patients with high-grade or advanced-stage disease are at significant risk of developing distant metastasis.93,106 Common sites of distant failure include lung, liver, and bone.96
Biologic Characteristics and Molecular Biology
Kidney
RCC occurs in both familial as well as sporadic forms. There are several hereditary syndromes associated with the development of RCC, including the von Hippel-Lindau syndrome, tuberous sclerosis, hereditary papillary renal carcinoma, Birt-Hogg-Dubé (BHD) syndrome, and hereditary renal carcinoma.107 The von Hippel-Lindau syndrome, an autosomal dominant disorder affecting 1 in 40,000 individuals, is caused by a mutation of the VHL tumor suppressor gene located on chromosome 3p. Silencing of the VHL gene by either somatic mutation or hypermethylation is believed to play a role in 50% to 60% of sporadic cases of clear cell RCC.108 Tuberous sclerosis is an autosomal dominant disorder affecting 1 in 10,000 individuals and results from mutations of either the TSC1 gene on chromosome 9q or the TSC2 gene on chromosome 6p.109 Hereditary papillary renal carcinoma is also an autosomal dominant disorder in which patients develop bilateral, multifocal lesions with associated germline mutations of the MET proto-oncogene located on chromosome 7q.110 Birt-Hogg-Dubé syndrome is a dominantly inherited predisposition to benign fibrofolliculomas and other skin and soft tissue tumors, including RCC. The gene for Birt-Hogg-Dubé syndrome has been mapped to chromosome 17p12-q11.2.111
Gene expression profiling has provided important information on the genetic basis of RCC.112 Genes that have been shown to have a role in renal carcinogenesis include the FHIT and RASSF1A tumor suppressor genes, transforming growth factor-beta receptor, hypoxia-inducible factors, PTEN, vascular endothelial growth factor, and carbonic anhydrases.113–119 Other genes that have been investigated but for which the data suggest little involvement in RCC include E-cadherin, TP53, BCL2, RB, c-MYC, and c-ERBB2.120–123
Renal Pelvis and Ureter
The karyotypic profile of UUT urothelial cancer has been shown to be similar to that of bladder cancers.124 Loss of the entire chromosome or partial loss of the short arm of chromosome 9 is a common finding, suggesting that it may be an early important event in tumorigenesis. Gains of DNA sequences are frequently observed in chromosome regions 1q21, 2p23, and 8q21.125 Overexpression of TP53, cyclin A, and cyclin E is seen in 24% to 30% of these tumors and is associated with worse prognosis.126–128 The expression of TP53 and caspase correlates with the pathologic stage and grade.129 The expression of survivin and the apoptotic index are associated with shorter disease-specific survival. Low level of CDKN1B is associated with tumor invasion and unfavorable prognosis.130
Clinical Manifestations, Patient Evaluation, and Staging
Kidney
Clinical Manifestations
RCC may present as an incidental finding on imaging or may demonstrate multiple presenting signs and symptoms caused by either local extension or metastatic disease. Because of these multiple symptoms, RCC has been called the “internist’s tumor.” Symptoms resulting from local tumor extension include hematuria, abdominal pain, and a flank mass. However, this “classic triad” of symptoms occurs in only about 10% of cases.90 Symptoms caused by metastatic disease include fever, weight loss, and night sweats. Two percent of male patients present with a varicocele, usually left sided, as a result of impaired drainage of the testicular vein.131
Paraneoplastic syndromes occur in about 30% of patients with RCC. Symptoms include hypertension, hypercalcemia, pyrexia, and hepatic dysfunction.132,133
Patient Evaluation
The widespread use of CT, MRI, and ultrasonography to evaluate the upper abdomen has significantly increased the incidental finding of RCC. Currently, 25% to 40% of diagnoses are made after the incidental detection of a renal mass.134,135 These tumors usually are smaller and, therefore, are more likely to be surgically resectable.136,137
The diagnosis of RCC is usually based on clinical and radiologic findings and pathologically confirmed at the time of nephrectomy. The staging evaluation includes a history and physical examination, complete blood cell count, serum chemistries, determination of lactate dehydrogenase level, urinalysis, chest radiography, and CT or MRI of the abdomen and pelvis (Table 54-1). If clinically indicated, bone scintigraphy and brain MRI may be considered. CT can predict tumor extent preoperatively in 90% of cases.138 MRI has been demonstrated to be superior to CT in determining inferior vena cava tumor extent.139 Magnetic resonance angiography can be used to evaluate vasculature preoperatively, especially when nephron-sparing surgery is being considered for patients with bilateral cancers.
TABLE 54-1 Diagnostic Evaluation/Algorithm for Kidney Carcinoma
Radiologic studies Computed tomography of the abdomen and pelvis or magnetic resonance imaging with intravenous gadolinium of the abdomen |
Staging
Two staging systems are used to define disease extent in RCC. Historically, Robson’s modification of the Flocks and Kadesky system was used.140 More recently, the AJCC Staging and End Results Reporting Classification has been used (Table 54-2).141 This system appears to be superior to the Robson system because it delineates more clearly local tumor extent and quantifies lymph node involvement.74
TABLE 54-2 American Joint Committee on Cancer Staging Classification System for Kidney and Ureteral Carcinoma, 7th Edition (2010)
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Kidney | |
| T1 | Tumor 7 cm or less in greatest dimension, limited to the kidney |
| T1a | Tumor 4 cm or less in greatest dimension, limited to the kidney |
| T1b | Tumor more than 4 cm but not more than 7 cm in greatest dimension, limited to the kidney |
| T2 | Tumor more than 7 cm in greatest dimension, limited to the kidney |
| T2a | Tumor more than 7 cm but less than or equal to 10 cm in greatest dimension, limited to the kidney |
| T2b | Tumor more than 10 cm, limited to the kidney |
| T3 | Tumor extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland and not beyond Gerota’s fascia |
| T3a | Tumor grossly extends into the renal vein or its segmental (muscle containing) branches, or tumor invades perirenal and/or renal sinus fat but not beyond Gerota’s fascia |
| T3b | Tumor grossly extends into the vena cava below the diaphragm |
| T3c | Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena cava |
| T4 | Tumor invades beyond Gerota’s fascia (including contiguous extension into the ipsilateral adrenal gland) |
| Ureteral/Renal Pelvis | |
| Ta | Papillary noninvasive carcinoma |
| Tis | Carcinoma in situ |
| T1 | Tumor invades subepithelial connective tissue |
| T2 | Tumor invades muscularis |
| T3 | (Renal pelvis only) Tumor invades beyond muscularis into peripelvic fat or renal parenchyma |
| T3 | (Ureter only) Tumor invades beyond muscularis into periureteric fat |
| T4 | Tumor invades adjacent organs or through kidney into perinephric fat |
| Regional Lymph Node (N)* | |
| Kidney | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in regional lymph node(s) |
| Ureteral/Renal Pelvis | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single regional lymph node, 2 cm or less in greatest dimension |
| N2 | Metastasis in a single lymph node, more than 2 cm but not more than 5 cm in greatest dimension; or multiple lymph nodes, none more than 5 cm in greatest dimension |
| N3 | Metastasis in a lymph node more than 5 cm in greatest dimension |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Stage Groupings | |
| Kidney | |
| I | T1N0M0 |
| II | T2N0M0 |
| III | T1-2N1M0 T3N0-1M0 |
| IV | T4, Any N0, M0 Any T, any N, M1 |
| Renal Pelvis and Ureteral Carcinoma | |
| 0a | TaN0M0 |
| 0is | TisN0M0 |
| I | T1N0M0 |
| II | T2N0M0 |
| III | T3N0M0 |
| IV | T4N0M0 Any T, N1-3, M0 Any T, any N, M1 |
* Laterality does not affect the N classification.
From Edge S, Byrd D, Compton C: AJCC Cancer Staging Manual. New York, Springer-Verlag, 2010.
Renal Pelvis and Ureter
Clinical Manifestations
The most common presenting symptom is gross or microscopic hematuria, which occurs in about 75% to 80% of patients. Flank pain is present in 27% to 35%.94,142 The pain may mimic that of ureteral calculus. Urinary symptoms such as frequency and dysuria are reported in 25% to 50% of patients. A palpable mass may be found in up to 10% of patients and is usually a hydronephrotic kidney. Constitutional symptoms such as malaise and weight loss are frequently seen in patients with extensive disease or metastases.
Patient Evaluation
Patients are evaluated with urine cytology, CT urography, and ureteroscopy. Cytology of voided urine detects 35% to 59% of UUT urothelial carcinomas and is more useful in higher-grade cancers.94 Urine specimens obtained by ureteral catheterization, or by washings of the upper tract, improve the positive yield of the study. Upper tract urine cytology is positive in up to 70% of patients.143 The positive predictive value of renal pelvis washing for high-grade cancer is 93% but is 43% for low-grade cancer.144 Radiologic findings of these tumors include a radiolucent filling defect or obstructive hydronephrosis. Ureteroscopy or percutaneous nephroscopy is performed if exfoliative cytology or radiologic studies show suspicious findings. Ureteroscopic biopsy provides the tissue diagnosis in up to 89% of cases.145
Additional studies include complete blood cell count and chemistries and chest radiography or chest CT. CT of the abdomen and pelvis is useful to assess the local extent of disease and intra-abdominal metastasis, although it is not a sensitive or specific test for nodal staging.146 If there is any concern about the function of the contralateral kidney, a renal scan should be performed before nephroureterectomy.
Staging
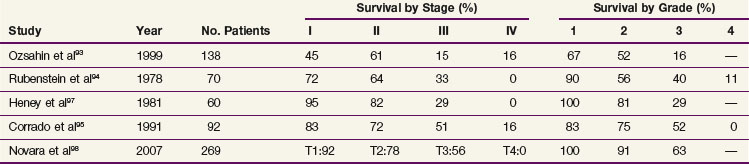
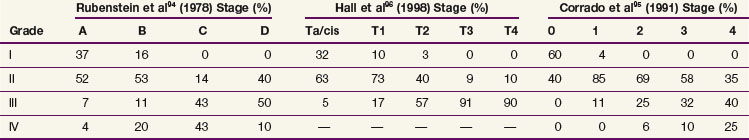
The current TNM staging system is listed in Table 54-2. Another staging system that is often used is the Jewett-Strong classification, which was originally used for bladder cancer: stage 0, limited to the mucosa; stage A, submucosal infiltration; stage B, invasion into but not through the muscle wall; stage C, invasion through the wall; stage D, lymphatic or distant metastasis.147 Tumor grade has a strong association with disease stage (Table 54-3). Both stage and tumor grade are important prognostic factors for survival. Table 54-4 illustrates the impact of stage and grade on the overall survival.
TABLE 54-3 Correlation between Tumor Grade and Stage of Disease for Renal Pelvis and Ureteral Cancer
Primary Therapy
Kidney
Surgery
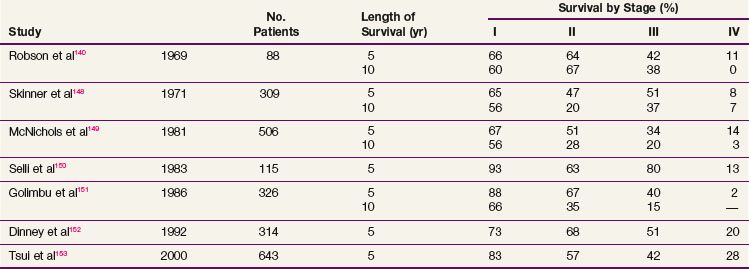
Complete surgical resection is the only possible curative treatment for RCC. A radical nephrectomy typically includes removal of the ipsilateral kidney with perinephric tissues including Gerota’s fascia and the ipsilateral adrenal gland. Survival results for treatment with standard open radical nephrectomy140,148–153 are delineated in Table 54-5. In a report of 643 patients,153 the 5-year DSS was 91% for those with stage I disease, 74% for those with stage II disease, 67% for those with stage III disease, and 32% for those with stage IV disease based on the 1997 TMN staging criteria. In a Mayo Clinic series of 1547 patients,154 the 10-year cause-specific survival (CSS) was 91% for T1, 70% for T2, 53% for T3a, 42% for T3b, and 43% for T3c tumors.
Removal of the adrenal gland is not required for RCC involving the middle or lower poles. However, upper pole cancers may necessitate adrenal gland removal with nephrectomy depending on the size, pathologic type, and extent of invasion of the renal tumor. Preoperative CT has demonstrated 99.6% specificity and a 94.4% negative predictive value in predicting adrenal gland involvement.155,156 In a review of 511 radical nephrectomies with adrenal gland removal,155 the incidence of renal cancer involvement with the adrenal gland was 5.7% and T1-2 tumors had an incidence of adrenal involvement of 0.6%.
A radical nephrectomy can include resection of hilar lymph nodes and occasionally includes regional lymph node dissection. Although regional lymph node dissection provides additional prognostic information, it has not been proven to prolong survival.74 The European Organization for Research and Treatment of Cancer (EORTC) conducted a phase III trial evaluating the role of lymph node dissection.157 Seven hundred seventy-two patients were randomized to a radical nephrectomy with or without a complete lymphadenectomy. All patients were clinically node negative based on preoperative CT. The incidence of positive lymph nodes was 4%. After a median follow-up of 12.6 years there were no differences in overall survival (OS), time to progression of disease, progression-free survival (PFS), or complications. One criticism of this trial is that 70% of patients were T1 or T2 category, suggesting a low risk of lymph node involvement. It is difficult to conclude whether patients with locally advanced tumors would benefit from a lymph node dissection. Researchers at the Mayo Clinic reported a review of 955 patients who underwent a lymph node dissection as part of a radical nephrectomy.158 High-grade tumor, presence of sarcomatoid features, tumor size greater than or equal to 10 cm, T3 category, and histologic tissue necrosis were significant predictors of lymph node-positive disease. The presence of these high-risk factors can help to determine which patients may benefit from an extensive lymph node dissection. Pantuck and colleagues159 retrospectively analyzed 900 patients with RCC and concluded that a lymph node dissection offers no survival advantage in clinically node negative patients but is associated with improved survival and improved response to immunotherapy in node-positive patients.
Partial nephrectomy, or nephron-sparing surgery, has been performed for small tumors. The initial indications for this surgery were presence of bilateral tumors or a cancer involving an anatomic or functional solitary kidney. Local recurrence rate was low (<6%), and this procedure has subsequently been performed in patients with small tumors (<4 cm).160 The incidence of local recurrence in these “electively” treated patients is 3% or less. Recent data from several institutions161,162 suggest larger tumors (>7 cm) can also be considered for nephron-sparing surgery. Patients undergoing nephron-sparing surgery have a decreased cumulative incidence of chronic renal insufficiency compared with patients who undergo radical nephrectomy.163,164
Laparoscopic radical nephrectomy and laparoscopic partial nephrectomy have become a commonly used surgical option for patients with RCC. Both pure and hand-assisted laparoscopic techniques for radical nephrectomy and nephron-sparing surgery achieve oncologic outcomes comparable to the standard open approach.165–169 Laparoscopic approaches have demonstrated advantages regarding blood loss, length of hospital stay, pain medication usage, cosmetics, and patient recovery compared with the standard open approach of radical nephrectomy.165,166
Ablation through radiofrequency heating and cryosurgical freezing are two frequently utilized techniques to obliterate suspicious small renal lesions.170,171 Employment of these techniques through an open, laparoscopic, or percutaneous approach has been demonstrated.170–172 A review of radiofrequency ablation and cryosurgical freezing with a laparoscopic or percutaneous approach173 revealed that laparoscopic ablation using either technique had a 0% to 15% relapse rate with a follow-up of 5 years. The percutaneous approach for ablation also demonstrated similar recurrence rates, but follow-up was only 2 years. The overall complication rate was 5%.
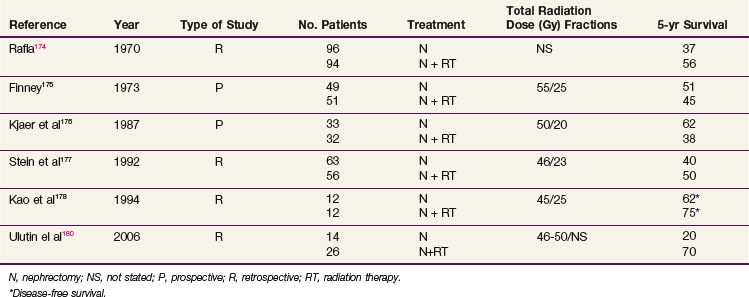
Adjuvant Irradiation
Although the use of postoperative irradiation has been evaluated both retrospectively and prospectively, its role remains controversial174,175–178 (Table 54-6). In a nonrandomized comparison of surgery with or without postoperative irradiation, Rafla174 found a 5-year OS advantage for 94 patients undergoing postoperative irradiation versus 96 patients treated by nephrectomy alone (56% vs. 37%). These data were confirmed in an expanded updated analysis. The 5-year OS was 38% (40/105) for patients who received postoperative irradiation versus 18% (24/135) for those undergoing nephrectomy alone.179
In a pseudo-randomized trial of 100 patients (randomization by odd vs. even date of birth), Finney175 found that incidence of distant metastases, local recurrence, and survival were not affected by the addition of postoperative radiation therapy (55 Gy in 25 fractions in 5 weeks). These results are difficult to interpret because randomization was not blinded or stratified by risk factors.
A small prospective, randomized study of postoperative radiation therapy was conducted by the Copenhagen Renal Cancer Study Group.176 Patients were randomized to nephrectomy alone (33 patients) versus nephrectomy and postoperative radiation therapy (32 patients). Postoperative irradiation consisted of 50 Gy in 20 fractions. The 5-year OS was 62% for nephrectomy alone versus 38% for nephrectomy and postoperative radiotherapy. Forty-four percent of the patients treated with radiation therapy had significant complications involving the stomach, duodenum, or liver that contributed to the death of 19% of these patients.
Four more recent retrospective studies evaluated the role of postoperative irradiation in patients at high risk for local recurrence after surgery for RCC, using multiple treatment fields and CT-based planning. Stein and associates177 evaluated 147 patients with localized RCC who underwent a nephrectomy. Fifty-six patients received postoperative irradiation with a median dose of 46 Gy. With a median follow-up of 19 months, there was no statistical difference in OS. However, there were more local recurrences in nonirradiated T3 patients (37% in nonirradiated patients vs. 11% in irradiated patients). In a follow-up descriptive study of patients treated with radiation after a nephrectomy, Gez and coauthors180 reported no benefit of postoperative radiation, even for T3 patients.
Kao and colleagues178 described 12 patients treated with nephrectomy alone and 12 patients treated with nephrectomy and postoperative irradiation, with a median dose of 45 Gy. After a median follow-up of 5 years, no difference in DFS was noted. However, the local failure rate was 30% for the nephrectomy-alone patients and 0% for those who had postoperative radiation therapy. In another retrospective study, Ulutin and colleagues181 found no difference in OS but a significant difference in DFS in favor of radiation therapy for 26 patients treated with postoperative irradiation compared with 14 patients treated with nephrectomy alone.
Rabinovitch and associates182 performed a patterns-of-failure analysis by CT on patients with localized RCC who underwent a radical or partial nephrectomy without adjuvant therapy. The risk of local failure after a gross resection was approximately 5%. Positive surgical margins and positive lymph nodes had a significant impact on local failure, increasing the rate of local failure to 21%. The risk of distant metastasis after gross resection was 26%. Lymph node involvement and renal vein involvement were significant prognosticators of distant metastases. Approximately half of patients who experienced a local relapse also experienced distant metastases concurrently or as the first site of relapse. Therefore the risk of micrometastatic disease at the time of surgery must be weighed against the potential benefit of local irradiation.
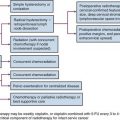
Adjuvant Chemotherapy, Immunotherapy, and Molecularly Targeted Treatment
Adjuvant therapy is given to patients with high risk of systemic disease relapse after curative surgery. Three separate clinical trials evaluating adjuvant interferon-alfa after nephrectomy183–185 failed to demonstrate a survival benefit. The Cytokine Working Group had conducted a high-dose intravenous bolus interleukin-2 (IL-2) trial versus observation in postnephrectomy patients,186 and it did not show any survival benefit. A different approach was studied by Jocham and colleagues187 using autologous tumor cell vaccine. Five hundred and fifty-eight patients with resected pT2-3b, pN0-3, M0 RCC were randomly assigned to vaccine compared with observation. At 4.5 years, PFS was 77% with the vaccine and 68% with observation. Its effect on OS would require longer follow-up. The role of targeted agents in the adjuvant setting is actively being investigated. One study in progress is the ECOG phase III study ECOG 2805, or the ASSURE study (Adjuvant Sorafenib or Sunitinib for Unfavorable risk REnal carcinoma).
Stereotactic Body Radiotherapy
The relative radioresistance of RCC and the previous experience with improvement in local control for patients treated with stereotactic radiosurgery for renal cell brain metastases have led to growing interests in the treatment of primary RCC with high-dose per fraction stereotactic body radiotherapy (SBRT). Beitler and colleagues188 reported on nine patients with nonmetastatic primary RCC who refused a nephrectomy. The majority of patients received 40 Gy in five fractions over 15 days. With a median follow-up of 26.7 months, four of nine patients survived. Only one patient had a local failure, which consisted of a new primary tumor in the unirradiated portion of the kidney. All surviving patients had an initial tumor size less than 3.4 cm and no clinical evidence of nodal disease, renal vein, or inferior vena caval extension or penetration of Gerota’s fascia. Wersall and associates189 treated eight patients with an inoperable primary or local inoperable recurrence. The median follow-up surpassed 58 months and only one of the patients experienced a local failure.
Svedman and colleagues190 conducted a single-institution phase II trial to evaluate the efficacy and safety of SBRT for RCC. Thirty patients with inoperable primary or metastatic RCC received treatment to 82 lesions. The most common dose fractionations were 32 to 40 Gy in four or five fractions of 8 Gy, 30 to 40 Gy in three or four fractions of 10 Gy, and 30 to 45 Gy in two or three fractions of 15 Gy. With a median follow-up time of 52 months for living patients and 22 months for deceased patients, local control was 98%. Sixteen of 28 patients experienced side effects, 96% of which were grade I or II.
Renal Pelvis and Ureter
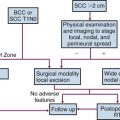
Surgery
Nephroureterectomy with removal of the bladder cuff is the standard treatment for patients with UUT cancer. Locoregional relapses occur in 9% to 15% of patients with low-grade, low-stage disease and in 30% to 50% of those with high-grade, high-stage disease. The removal of the bladder cuff is recommended because ureteral stump recurrence is noted in 11% to 55% of patients if the stump is left in place.147,191,192 Patients with T2-4 disease should have lymphadenectomy. Nodal status is a significant prognostic factor and may be used to guide the decision for adjuvant treatment. For selected patients with distal ureteral cancers, distal ureterectomy and lymphadenectomy with reimplantation of ureter may be an option.
Laparoscopic nephroureterectomy has been shown to achieve similar disease control in many patients, with decrease in hospital stay and shorter recovery time, as compared with open nephroureterectomy.193–197 In a report of 115 patients,195 port-site metastasis occurred in one patient (0.9%). Minimally invasive surgery with robot-assisted ureterectomy and ureteral reconstruction appears to be safe and feasible in some patients, but the oncologic effectiveness of this procedure needs to be confirmed.198
There are conflicting reports96,97,199,200,201 about the efficacy of more conservative nephron-sparing surgery or partial ureterectomy, which often can be done endoscopically. One study reported up to 62% recurrence, and careful follow-up is imperative for these patients. The selection criteria usually include low-grade and low-stage disease, superficial disease, small lesion size (<1.0 to 1.5 cm), absence of multifocal disease, solitary kidney, bilateral disease, or major medical comorbidities. These patients would need to be followed very closely so that nephroureterectomy can be offered if recurrence is detected.
Primary Irradiation
Primary irradiation is rarely used as initial treatment. The few published reports using primary irradiation are mostly case reports or small series. Some patients with gross residual disease after nephroureterectomy or who have recurrent disease are treated with external beam irradiation. In a series with 19 patients with unresectable disease,191 the median survival was 11 months for the 11 patients treated with radiation therapy versus 4 months for those who did not receive radiation. Two patients were disease free after receiving 45 Gy and 50.4 Gy, respectively, for gross residual disease after surgery. Batata and coauthors147 reported one of eight patients had long-term disease control after EBRT for gross residual disease after surgery, using doses of 10 Gy in 5 fractions to 60 Gy in 35 fractions.
Adjuvant Irradiation
The risk of local relapse correlates with the stage of disease and tumor grade and can be up to 45% despite radical surgery. In a study by Cozad and colleagues,191 the 5-year local control rate was 90% for grade 1 and 2 tumors and 41% for grade 3 and 4 tumors, respectively. For stage I and II disease, the 5-year local control rate was 83%, compared with 52% for stage III disease. About half of the local relapses presented initially as isolated site of relapse. Distant metastasis occurred in 19% of patients with stage I and II disease and in 53% with stage III disease. Distant metastasis was the predominant site of initial relapse for stage III disease. The risk of lymph node metastasis also increases with disease stage and tumor grade.103 The pattern of failure demonstrates that patients with high-stage or high-grade disease have significant risks of both distant and local relapse, and many of them may have locoregional failure as the initial site of relapse.
Few studies have been published to evaluate the role of adjuvant irradiation in the management of UUT cancers.147,202,203 In a study by Cozad and colleagues,202 26 patients with T3 or T4 N0/+ disease had radical surgery, 9 of whom received adjuvant irradiation to a median dose of 50 Gy. Local failure occurred in 9 of 17 without and 1 of 9 with adjuvant irradiation (p = .07). The effect of adjuvant irradiation was seen only in high-grade tumors, because no local relapse developed in patients with low-grade disease regardless of whether adjuvant therapy was given. Metastasis developed in 4 of 9 and 8 of 17 patients with and without radiation therapy, respectively. Five-year OS was 44% with, and 24% without, adjuvant irradiation, respectively (p = .23). In a study by Brookland and Richter,203 adjuvant irradiation was given to 11 of 23 patients who had tumor grade 3 or 4, or pathologic stage C or D. The median dose was 50 Gy to the ureteropelvic bed, with or without inclusion of the para-aortic nodes. For the nonirradiated group, the median survival was 26 months and the local relapse rate was 45%, compared with 35 months and 11%, respectively, for those who received adjuvant irradiation. Other studies have questioned the benefits of adjuvant irradiation. In a series of 138 patients,93 45 received postoperative irradiation with a median dose of 50 Gy. The 5-year OS was 21% for those who received postoperative irradiation, compared with 33% for those who did not. However, patients who received postoperative irradiation had significantly higher T category and tumor grade. Maulard-Durdux and colleagues106
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree