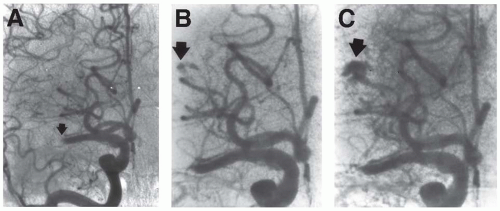
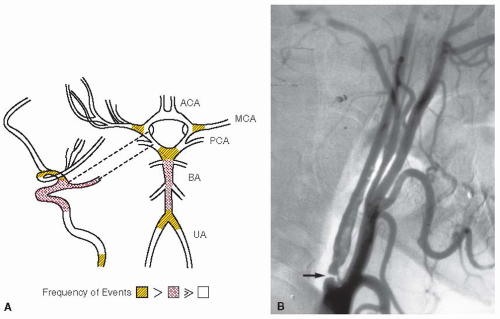
Antiplatelet Agents
Aspirin (Acetylsalicylic Acid). In ischemic cerebrovascular disease, ASA can reduce the incidence of TIAs and subsequent ischemic strokes. Despite the large number of prospective evaluations of ASA, a small number of well-conducted level I studies “drive” the efficacy of ASA in the carotid artery territory (
Table 93.2).
The Aspirin in Transient Ischemic Attacks (AITIA) study— which randomized patients with a 3-month history of TIAs, who were not considered candidates for carotid endarterectomy, to ASA (1,300 mg/day) or to placebo—demonstrated a reduction in stroke and vascular death at 6 months with ASA exposure.
130 There was a considerable decrease in the combined outcomes of recurrent TIAs, cerebral/retinal infarction, and death among those receiving ASA, but no reduction by life-table analysis at the 24-month follow-up and no reduction in stroke incidence. In the Canadian Cooperative Study Group trial, patients with a history of TIAs were randomized to ASA (1,300 mg/day), sulfinpyrazone (800 mg/day), and the combination ASA/sulfinpyrazone, or placebo.
131 Patients who received ASA had a significant decrease in the incidence of stroke and death compared to sulfinpyrazone or placebo.
131 The risk reduction for stroke and death with ASA was most significant for men. Among patients with a history of TIAs in the double-blind “Accidents, Ischemiques Cerebraux Lies a l’ Athersclerose (AICLA)” trial who were randomized to ASA (990 mg/day), the combination of ASA (990 mg/day) with dipyridamole (225 mg/day), or placebo, those who received ASA or the combination demonstrated a reduced incidence of stroke, MI, and death compared to patients who received placebo.
132 Efficacy in the combined outcome seemed to be associated with an ASA dose range of 990 to 1,300 mg/day.
However, the ASA doses used were associated with considerable side effects. This was confirmed by the UK-TIA aspirin trial, which randomized 2,435 individuals with TIAs or minor ischemic stroke to “high dose” ASA (1,200 mg/day), “low-dose” ASA (300 mg/day), or placebo.
15 The 4-year incidence of nonfatal MI, nonfatal major stroke, and death was significantly reduced by 18% in a dose-dependent manner in patients who received ASA. But, gastrointestinal side effects were more common with the high-dose regimen, and excess mortality was attributed to intracranial hemorrhage in the ASA-treated groups. In a separate study, a decrease in major hemorrhagic events, including fatal intracerebral hemorrhage, was associated with lower ASA doses (30 vs. 283 mg/day).
133The Swedish Aspirin Low-Dose Trial (SALT) Collaborative Group reported an 18% risk reduction of stroke and death among TIA patients who started ASA (75 mg/day) within 1 to 4 months of their initial symptoms compared to patients who received placebo.
134 A 16% to 20% reduction in the risk of stroke, frequent TIAs, and MI was also observed.
The results of those trials, and meta-analyses of all clinical series, suggest that even low doses of ASA benefit early stroke and vascular mortality in patients with a history of TIAs or minor ischemic events.
Combination ASA/Dipyridamole. Clinical use of the combination of ASA with dipyridamole has been based upon positive interactions between the agents in both preclinical experiments and in clinical platelet survival studies.
135,136,137 The efficacy of this combination, however, has been challenged.
136 Dipyridamole (400 to 800 mg/day) alone did not affect stroke incidence or related mortality compared with placebo in one limited double-blind, level I randomized trial.
138
The European Stroke Prevention Study (ESPS) Group demonstrated a 33% reduction in risk of stroke and death with ASA (975 mg/day) and dipyridamole (225 mg/day) compared to placebo.
139 The three-arm “AICLA” study also demonstrated the benefits from the combination of ASA with dipyridamole over placebo (but no difference with ASA alone) for patients with TIAs when the outcome events of stroke, MI, and mortality were combined.
132 However, there was no difference in stroke or mortality between the combination of ASA (1,300 mg/day) and dipyridamole (300 mg/day) and ASA alone in the American-Canadian Cooperative Study Group.
140On the basis of experimental evidence suggesting that sustained release extended-release dipyridamole (ER-DP) could produce elevated plasma adenosine levels, the relative efficacy of the fixed combination of low-dose ASA (50 mg/day)/ER-DP (400 mg/day) was tested in the ESPS-2 against ER-DP, ASA, or placebo. At 2-year follow-up, low-dose ASA alone and dipyridamole alone (relative risk reduction [RRR] for stroke, 15.8% and 17.7%, respectively) and the combination (RRR, 36.7%) were found to be superior to placebo in limiting stroke, death, or both for individuals with a history of TIAs or recent stroke.
77,141The European/Australian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) Group examined the impact of combination ASA (30 to 375 mg/day)/dipyridamole (400 mg/day) or ASA alone on vascular death, nonfatal stroke, nonfatal MI, or major hemorrhage in patients presenting with TIA or minor ischemic stroke.
142,143,144 With a mean follow-up of 3.5 years, the combination of ASA/dipyridamole was superior to ASA alone (HR = 0.80, 95% CI = 0.66 to 0.98) in reduction of the primary outcome events. While headache occurred in the combination, overall ASA/dipyridamole improved outcome in this trial and produced a decrease in ischemic events.
143 The greater efficacy of the combination supports its use in the clinical setting.
In a separate study, clopidogrel was prospectively compared to the combination of aspirin/ER-DP with or without the angiotensin receptor blocker telmisartan in the Prevention Regimen for Effectively avoiding Second Strokes (PRoFESS) study of first recurrent stroke in a 2 × 2 factorial design.
145 In total, 20,332 patients were randomized within 120 days of the initial event and followed for a mean of 2.5 years. There was no difference in the incidence of recurrent strokes between the two groups, although there were more hemorrhagic events in the aspirin/ER-DP arms. This trial did not meet the predefined criteria for noninferiority.
Sulfinpyrazone. The Canadian Cooperative Study Group compared ASA, sulfinpyrazone (800 mg/day), a uricosuric agent with antiplatelet effect, and placebo but found no significant difference in the incidence of stroke and mortality between sulfinpyrazone and placebo over 2.2 years.
131 Sulfinpyrazone (800 mg/day) and placebo were also tested in a double-blind, cross-over trial.
146 A nonsignificant reduction in TIAs and in stroke and mortality at 4-month follow-up was recorded in patients receiving sulfinpyrazone. Another level I study comparing sulfinpyrazone (800 mg/day) and ASA (1,000 mg/day) in individuals with TIAs concluded that there was a higher incidence of stroke, MI, and vascular death in the sulfinpyrazone cohort at 11-month follow-up.
147
Adenosine Diphosphate Receptor Antagonists. Thienopyridines irreversibly block the adenosine diphosphate platelet receptor P2Y
12 and inhibit other downstream events, thereby blocking platelet activation. Ticlopidine found benefit in patients with TIA/minor stroke
148 and in prevention of secondary stroke (see below). Ticlopidine (500 mg/day) was associated with a statistically significant 12% reduction in the risk of stroke and death from any cause over ASA (1,300 mg/day) and a 21% reduction in the risk of secondary outcomes of stroke and stroke-related death following TIAs. Reversible leukopenia occurred in 0.8% of patients taking ticlopidine, whereas gastrointestinal symptoms were more common in patients receiving ASA. Intracerebral hemorrhage occurred equally in both groups. Because of concerns about the frequency of thrombotic thrombocytopenic purpura, ticlopidine has been supplanted by the related thienopyridine clopidogrel.
In summary, ASA reduces the risk of stroke, MI, and mortality in individuals with a history of TIAs or minor stroke. This risk reduction is obviously not dose dependent. The fixed combination of low-dose ASA/ER-DP also produces a substantial risk reduction. Ticlopidine was more effective than ASA in preventing stroke and mortality after TIAs in one study but is seldom used now because of unwanted side effects. When used alone, dipyridamole and sulfinpyrazone have little demonstrated benefit.
149,150
Anticoagulation
Changes in plasma thrombin concentration and FDP levels occur following ischemic stroke that suggest that interruption of coagulation may be beneficial. In the 1960s and 1970s, clinical experience with anticoagulation in patients with TIAs or minor stroke was limited.
151,152,153,154 Uncontrolled reports suggested that heparin could decrease the incidence of basilar artery TIAs.
155,156 Fisher observed a transient reduction in the incidence of TIAs in 97% (of 29) patients treated with anticoagulants, with TIAs returning in 60% of those in whom anticoagulation was discontinued.
157 Two studies reported that long-term anticoagulation decreased the incidence of strokes and/or vascular death among patients with recent TIAs
158,159; a third study showed that despite a decrease in TIA frequency, there was no decrease in the incidence of stroke or vascular death.
160 Two other studies failed to show superiority of anticoagulation over its comparator for the incidence of stroke or death.
161,162 In a small Medical Research Council (MRC)-sponsored trial of phenindione, patients with TIA receiving fixed “low-dose” anticoagulation had a lower incidence of vascular events, stroke, and death than those patients receiving an adjusted “high-dose” regimen.
163 Only the Cerebral Embolism Study Group trial indicated a benefit from immediate anticoagulation for cardiogenic embolism.
164Subsequently, there have been few controlled prospective randomized level I studies. The Warfarin-Aspirin Recurrent Stroke Study (WARSS) found no difference in outcome between TIA and stroke patients treated with oral anticoagulation under tight control compared to patients treated with ASA.
165 There was no increase in intracerebral hemorrhage in the anticoagulated group, most probably because of the rigid control of the international normalized ratio (INR).
165The risk of intracerebral hemorrhage increases with age in patients treated with oral anticoagulants which inhibit vitamin K activity.
166,167 With careful monitoring and dose adjustment, this risk can be reduced.
167 The Stroke Prevention in Reversible Ischemic Trial
168 was terminated because of an unacceptably high incidence of intracranial hemorrhages in the group at a high target INR of 3.0 to 4.5.
In summary, excluding individuals with AF or cardiac valvular prostheses, the results of various small studies do not convincingly support a role for anticoagulation in patients with TIA or minor stroke, considering the risk of hemorrhage. The risk of intracerebral hemorrhage is increased for patients receiving oral anticoagulants at INRs in excess of 3.0 to 4.5.