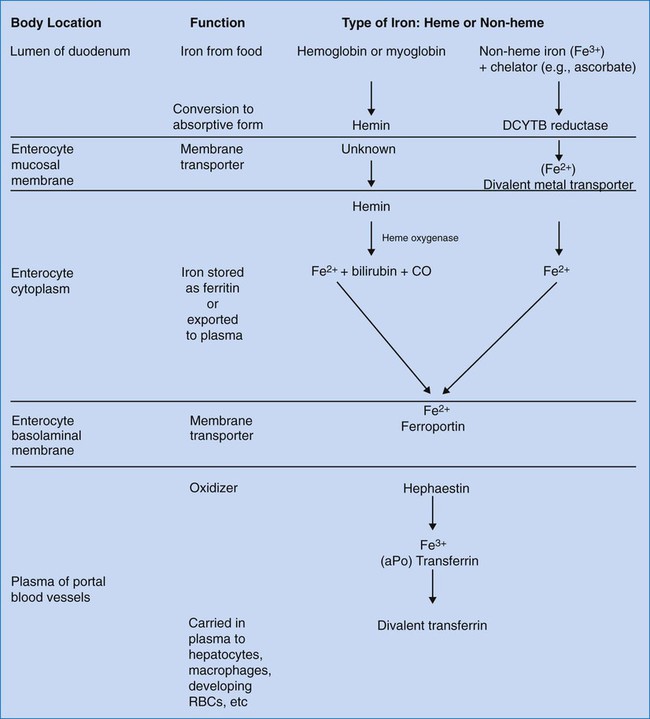
After completion of this chapter, the reader will be able to: 1. Discuss the role of iron as an essential nutrient for human survival. 2. List the sites in which iron is distributed in the body and state the approximate amount in each site. 3. State the minimum daily intake of iron required at various ages for men, women, and children. 4. Describe the mechanism of iron absorption and distinguish between the absorption of heme and nonheme iron. 5. State the site of iron absorption. 6. Describe transferrin, transferrin receptor, hepcidin, hemosiderin, and ferritin, including function and regulation. 7. Diagram the transport of iron from ingestion to incorporation into heme. 8. Define sideroblast, siderocyte, and siderosome. 9. Discuss regulation, excretion, transport, and storage of iron in the body. 10. Identify and discuss the laboratory tests currently used to assess iron status in the body. Iron in its cationic bivalent ferrous (Fe2+) and trivalent ferric (Fe3+) states is essential for the life of all organisms, plant and animal.1 In humans, 70% of total body iron is transported in blood noncovalently bound in the ferrous state to the heme portion of hemoglobin, where it binds, transports, and releases oxygen (O2, Table 11-1).2 In mitochondria, ferrous ion is transferred to protoporphyrin IX (see Figure 10-5) to form heme. Four heme molecules become bound, one each, to four globin chains to produce tetrameric hemoglobin. Ferrous iron likewise binds to myoglobin, the O2 transport molecule in muscles. The primary and secondary structures of myoglobin resemble hemoglobin, however, myoglobin is monomeric, and myoglobin oxygen binding is irreversible. Approximately 5% of total human body iron is bound to myoglobin. TABLE 11-1 Iron Compartments in Normal Humans Iron overload results from increased absorption owing to genetic predisposition or repeated blood transfusions and produces potentially fatal heart and liver disease. Evolution has given humans a mechanism for absorbing dietary iron efficiently but not for eliminating excess iron effectively. Disorders of iron metabolism are discussed in Chapter 19. Although many foods have high iron content, the iron may be minimally bioavailable. The bioavailability of iron depends on its chemical form and the presence of non-iron foods that promote or inhibit absorption. An average American diet may provide 10 to 20 mg of iron/day, but only 1 to 2 mg/day is absorbed. Iron is absorbed in two forms: heme and nonheme. Heme-bound iron, mainly from meat, is absorbed more efficiently than nonheme, inorganic iron and in a different manner.3 Heme iron is present in hemoglobin, myoglobin, and heme-containing enzymes. Approximately 5% to 35% of heme iron is absorbed as hemin (iron-containing porphyrin). Nonheme iron, found in nonmeat sources such as legumes and leafy vegetables, accounts for approximately 90% of dietary iron, but only 2% to 20% of it is absorbed, depending on the iron status of the individual and the ratio of dietary enhancers and inhibitors.1 Ascorbate, citrate, and other organic acids and amino acids enhance absorption of nonheme iron by the formation of soluble chelates. Cooking in iron pots increases the amount of iron consumed. Substances that interfere with nonheme iron absorption include phytates, polyphenols, phosphates, oxalates, and calcium.4,5 The duodenum and upper jejunum are sites of maximal absorption of iron. For transport of oxygen in Hb, iron must be in the ferrous form (Fe2+). To be absorbed from food, iron must be in the form of heme iron (Fe2+) or converted from ferric nonheme iron to the soluble ferrous form by a duodenum-specific cytochrome b–like protein, DCYTB.6 Uptake of heme iron occurs on heme carrier protein 1, located on the apical membrane of the duodenal enterocyte.7 Heme iron binds to the enterocyte in the mucosal epithelium and is internalized (Figure 11-1). Here the enzyme heme oxygenase degrades heme to produce ferrous iron, carbon monoxide, and bilirubin-IXa. Ferrous iron is transported across the duodenal epithelium bound to the apical divalent metal transporter 1 (DMT1). The ferrous iron is carried to the basolateral membrane (base and sides of the enterocyte membrane), from which it is exported to the portal circulation, a process mediated by ferroportin, a basolateral transport protein. Ferroportin works in conjunction with a copper-containing iron oxidase known as hephaestin. Hephaestin may facilitate iron egress by reoxidation of ferrous to ferric iron.6 The trivalent (ferric) iron must be bound to transferrin to be transported through the circulation. Some iron remains in the enterocytes as ferritin and is released to the circulation over a few hours. Enterocyte-stored ferritin iron is excreted when the cells are exfoliated in the stool. Hepcidin, an antimicrobial peptide produced in the liver, seems to act as a negative regulator of intestinal iron absorption. It also suppresses release from macrophages. Hepcidin binds to the ferroportin receptor, causing degradation of ferroportin and trapping iron in the intestinal cells. Hepcidin synthesis rises when transferrin is carrying its maximum capacity of iron (transferrin saturation of more than 50% in females or 60% in males), and diminishes when iron saturation is low.7–9 The role of hepcidin in the anemia of chronic inflammation is discussed in Chapter 19. Transferrin transports ferric iron (Fe3+) to hematopoietic and other tissues, where it is bound by cell membrane transferrin receptors (Figure 11-2). Transferrin receptors are expressed in larger amounts on normoblasts and rapidly dividing cells, whether normal or malignant, but not on highly differentiated cells such as reticulocytes.10 Transferrin is taken into the cell by endocytosis.
Iron Metabolism
Case Study
Compartment
Percent of Total Body Iron
Iron Content (mg/kg Body Weight)
Hemoglobin iron
∼65%
2.6
Storage iron: hemosiderin, ferritin
∼25%
14.2
Myoglobin iron
∼5%
1.0
Transferrin
∼0.1%
0.003
Other compartments:
∼3.6%
0.140
Peroxidase, catalase, cytochromes, riboflavin enzymes
Dietary Iron
Iron Absorption and Excretion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine

 years. The investigators collected comparable data from nondonors. Of the donors, 10 women and 6 men took a dietary supplement providing approximately 20 mg of iron per day. In addition, mean iron dietary intake was 16.4 mg/day for the women and 19.9 for the men. Over the period of the study, mean iron stores in women decreased from 12.53 to 1.14 mg/kg of body weight. Mean iron stores in men declined from 12.45 to 1.92 mg/kg. Nondonors’ iron stores remained unchanged. Based on Hb and Hct results, no donors became anemic. There was no statistically significant difference in iron stores between the men who took supplements and those who did not, although a difference was seen for the women. Total iron losses over 80 days, the average interval between donations, were calculated to be 4.32 mg/kg for the women and 3.93 mg/kg for the men. As iron stores decreased, the calculated iron absorption rose to 3.55 mg/day for the women and 4.10 mg/day for the men.
years. The investigators collected comparable data from nondonors. Of the donors, 10 women and 6 men took a dietary supplement providing approximately 20 mg of iron per day. In addition, mean iron dietary intake was 16.4 mg/day for the women and 19.9 for the men. Over the period of the study, mean iron stores in women decreased from 12.53 to 1.14 mg/kg of body weight. Mean iron stores in men declined from 12.45 to 1.92 mg/kg. Nondonors’ iron stores remained unchanged. Based on Hb and Hct results, no donors became anemic. There was no statistically significant difference in iron stores between the men who took supplements and those who did not, although a difference was seen for the women. Total iron losses over 80 days, the average interval between donations, were calculated to be 4.32 mg/kg for the women and 3.93 mg/kg for the men. As iron stores decreased, the calculated iron absorption rose to 3.55 mg/day for the women and 4.10 mg/day for the men.