Chapter Outline
Components of normal haemostasis 367
The blood vessel 367
Platelets 368
Blood coagulation 369
Inhibitors of coagulation 372
The fibrinolytic system 372
General approach to investigation of haemostasis 372
Notes on equipment 374
Pre-analytical variables including sample collection 375
Calibration and quality control 376
Reference standard (calibrator) 376
Calibration of standard pools and suggested calibration procedure 376
Control plasma 377
Variability of coagulation assays 377
Performance of coagulation tests 377
Handling of samples and reagents 377
Eliminating a time trend 377
Assay monitoring and end-point detection 377
Analysis time over 379
Turbidity level over 379
Commonly used reagents 380
The ‘clotting screen’ 380
Prothrombin time 380
Activated partial thromboplastin time 381
Thrombin time 382
Measurement of fibrinogen concentration 383
Fibrinogen assay (Clauss technique) 383
Platelet count 383
Interpretation of first-line tests 383
Second-line investigations 384
Correction tests using the prothrombin time or activated partial thromboplastin time 384
Correction tests using the thrombin time 385
Reptilase (batroxobin) or ancrod time 386
Investigation of a bleeding disorder resulting from a coagulation factor deficiency or defect 386
General principles of parallel line bioassays of coagulation factors 386
Assays based on the prothrombin time 387
Assays based on the activated partial thromboplastin time 387
Monitoring replacement therapy in coagulation factor defects and deficiencies 389
Investigation of a patient with a circulating anticoagulant (inhibitor) 389
Circulating inhibitor (anticoagulant) screen based on the activated partial thromboplastin time 389
Quantification of factor VIII inhibitors 390
Investigation of a patient with suspected afibrinogenaemia, hypofibrinogenaemia or dysfibrinogenaemia 392
Fibrinogen estimation (dry clot weight) 392
Defects of primary haemostasis 392
Investigation of the vascular disorders of haemostasis 392
Laboratory tests of platelet–von Willebrand factor function 393
Investigation of suspected von Willebrand disease 393
Enzyme-linked immunosorbent assay for von Willebrand factor antigen 393
von Willebrand factor antigen immunoturbidimetric assay 395
Ristocetin cofactor assay 395
Assay using fresh platelets 395
Assay using formalin-fixed platelets 396
Automated assays of von Willebrand factor platelet-binding function 397
Collagen-binding assay (ELISA) 397
Investigation of a suspected disorder of platelet function, inherited or acquired 398
Laboratory investigation of platelets and platelet function 398
Platelet aggregation 399
Further investigation of platelet function 403
Assays of factor XIII activity 404
Disseminated intravascular coagulation 405
Detection of fibrinogen/fibrin degradation products using a latex agglutination method 405
Screening tests for fibrin monomers 406
Detection of crosslinked fibrin D-dimers using a latex agglutination method 406
Investigation of carriers of a congenital coagulation deficiency or defect 406
Components of normal haemostasis
The haemostatic mechanisms have several important functions: (1) to maintain blood in a fluid state while it remains circulating within the vascular system; (2) to arrest bleeding at the site of injury or blood loss by formation of a haemostatic plug; (3) to limit this process to the vicinity of the damage and (4) to ensure the eventual removal of the plug whilst healing is completed. Normal physiology thus constitutes a delicate balance between these conflicting tendencies, and a deficiency or exaggeration of any one may lead to either thrombosis or haemorrhage. There are at least five different components involved: blood vessels, platelets, plasma coagulation factors and their inhibitors and the fibrinolytic system. In this chapter a brief review of normal haemostasis is presented followed by a discussion on the general principles of basic tests used to investigate haemostasis and bleeding disorders.
The blood vessel
General structure of the blood vessel
The blood vessel wall has three layers: intima, media and adventitia. The intima consists of endothelium and subendothelial connective tissue and is separated from the media by the elastic lamina interna. Endothelial cells form a continuous monolayer lining all blood vessels. The structure and the function of the endothelial cells vary according to their location in the vascular tree, but in their resting state they all share three important characteristics: they are ‘nonthrombogenic’ (i.e. they promote maintenance of blood in its fluid state), they play an active role in supplying nutrients to the sub-endothelial structures and they act as a barrier to cells, macromolecules and particulate matter circulating in the bloodstream. The permeability of the endothelium may vary under different conditions to allow various molecules and cells to pass.
Endothelial cell function
The luminal surface of the endothelial cell is covered by the glycocalyx, a proteoglycan coat. It contains heparan sulphate and other glycosaminoglycans, which are capable of activating antithrombin, an important inhibitor of coagulation enzymes. Tissue factor pathway inhibitor (TFPI) is present on endothelial cell surfaces, mostly in a truncated form covalently linked to a glycophosphoinositol (GPI) anchor (TFPIbeta) although some full length TFPI (TFPIalpha) is also present and noncovalently bound. Endothelial cells express a number of coagulation-active proteins that play an important regulatory role, such as thrombomodulin and the endothelial protein C (PC) receptor. Thrombin diffusing away from the site of injury is rapidly bound to thrombomodulin which can then activate PC bound to the endothelial protein C receptor (EPCR) and a carboxypeptidase which inhibits fibrinolysis (discussed later). Thrombin also stimulates the endothelial cell to produce tissue plasminogen activator (tPA). The endothelium can also synthesise protein S, the cofactor for PC. Finally, endothelium produces von Willebrand factor (VWF) which is essential for platelet adhesion to the subendothelium and stabilises factor VIII (FVIII) within the circulation. VWF is both stored in specific granules called Weibel Palade bodies and secreted constitutively, partly into the circulation and partly toward the subendothelium where it binds directly to collagen and other matrix proteins. The expression of these and other important molecules such as adhesion molecules and their receptors are modulated by inflammatory cytokines. The lipid bilayer membrane also contains adenosine diphosphatase (ADPase), an enzyme that degrades adenosine diphosphate (ADP), which is a potent platelet agonist (see p. 401). Many of the surface proteins are found localised in the specialised lipid rafts and invaginations called caveolae which may provide an important level of regulation.
The endothelial cell participates in vasoregulation by producing and metabolising numerous vaso-active substances. On one hand it metabolises and inactivates vasoactive peptides such as bradykinin, on the other hand, it can also generate angiotensin II, a local vasoconstrictor, from circulating angiotensin I. Under appropriate stimulation the endothelial cell can produce vasodilators such as nitric oxide (NO) and prostacyclin or vasoconstrictors such as endothelin and thromboxane. These substances have their principal vasoregulatory effect via the smooth muscle but also have some effect on platelets.
The subendothelium consists of connective tissues composed of collagen (principally types I, III and VI), elastic tissues, proteoglycans and noncollagenous glycoproteins, including fibronectin and VWF. After vessel wall damage has occurred, these components are exposed and are then responsible for platelet adherence. At low shear platelets can bind to collagen but in practice this appears to be largely mediated by VWF binding to collagen. VWF bound to collagen undergoes a conformational change and platelets are captured via their surface membrane glycoprotein Ib binding to VWF. Platelet activation follows, and a conformational change in glycoprotein IIbIIIa allows further, more secure, binding to VWF via this receptor as well as to fibrinogen.
Vasoconstriction
Vessels with muscular coats contract following injury thus helping to arrest blood loss. Although not all coagulation reactions are enhanced by reduced flow, this probably assists in the formation of a stable fibrin plug by allowing activated factors to accumulate to critical concentrations. Vasoconstriction , also occurs in the microcirculation in vessels without smooth muscle cells. Endothelial cells themselves can produce vasoconstrictors such as angiotensin II. In addition, activated platelets produce thromboxane A 2 (TXA 2 ), which is a potent vasoconstrictor.
Platelets
Platelets are small fragments of cytoplasm derived from megakaryocytes. On average they are 1.5 to 3.5 μm in diameter but may be larger in some disease states. They do not contain a nucleus and are bounded by a typical lipid bilayer membrane. Beneath the outer membrane lies the marginal band of microtubules, which maintain the shape of the platelet and depolymerise when aggregation begins. The central cytoplasm is dominated by the three types of platelet granules: the δ (dense) granules, α granules and lysosomal granules. The contents of these various granules are detailed in Table 18-1 . Finally there exist the dense tubular system and the canalicular membrane system; the latter communicates with the exterior.
| Dense (δ) Granules | α Granules | Lysosomal Vesicles |
|---|---|---|
| ATP | PF4 | Galactosidases |
| ADP | VWF | Fucosidases |
| Calcium | β-thromboglobulin | Hexosaminidase |
| Serotonin | Fibrinogen | Glucuronidase |
| Pyrophosphate | Factor V | Cathepsin |
| P selectin (CD62) | Thrombospondin | Glycohydrolases |
| Transforming growth factor-beta (1) | Fibronectin PDGF PAI-1 | + others |
| Catecholamines (epinephrine/norepinephrine) | Histidine-rich glycoprotein α 2 Macroglobulin | |
| GDP/GTP | Plasmin inhibitor | |
| P selectin (CD62) |
The platelet membrane is the site of interaction with the plasma environment and with the damaged vessel wall. It consists of phospholipids, cholesterol, glycolipids and at least nine glycoproteins (GP), named GPI to GPIX. The membrane phospholipids are asymmetrically distributed, with sphingomyelin and phosphatidylcholine predominating in the outer leaflet and phosphatidyl-ethanolamine, -inositol and -serine in the inner leaflet. After platelet activation, phosphatidylserine is exposed on the outer membrane surface to support assembly of coagulation factor complexes.
The contractile system of the platelet consists of the dense microtubular system and the circumferential microfilaments, which maintain the disc shape. Actin is the main constituent of the contractile system, but myosin and a regulatory calcium-binding protein, calmodulin, are also present.
Platelet function in the haemostatic process
The main steps in platelet functions are adhesion, activation with shape change and aggregation. When the vessel wall is damaged, the subendothelial structures, including basement membrane, collagen and microfibrils, are exposed. VWF binds to collagen and microfibrils and then captures platelets via initial binding to platelet GPIb, resulting in an initial monolayer of adherent platelets. Binding via GPIb initiates activation of the platelet via a G-protein mechanism. Once activated, platelets immediately change shape from a disc to a sphere with numerous projecting pseudopods. After adhesion of a single layer of platelets to the exposed subendothelium, platelets stick to one another to form aggregates. Fibrinogen, fibronectin, further VWF released from platelets and the glycoprotein IbIX and IIbIIIa complexes are essential at this stage to increase the cell-to-cell contact and facilitate aggregation. Positive feedback is provided by platelet release of ADP, 5-hydroxytryptamine (5HT) and thromboxane. In areas of nonlinear blood flow, such as may occur at the site of an injury, locally damaged red cells release ADP, which further activates platelets.
Platelet aggregation
Platelet aggregation may occur by at least two independent but closely linked pathways. The first pathway involves arachidonic acid metabolism. Activation of phospholipase enzymes (PLA 2 ) releases free arachidonic acid from membrane phospholipids (phosphatidyl choline). About 50% of free arachidonic acid is converted by a lipo-oxygenase enzyme to a series of products including leucotrienes, which are important chemoattractants of white cells. The remaining 50% of arachidonic acid is converted by the enzyme cyclooxygenase into labile cyclic endoperoxides, most of which are in turn converted by thromboxane synthetase into TXA 2 . TXA 2 has profound biological effects, causing secondary platelet granule release and local vasoconstriction, as well as further local platelet aggregation via the second pathway below. It exerts these effects by raising intracellular cytoplasmic free calcium concentration and binding to specific granule receptors. TXA 2 is very labile with a half-life of less than 1 min before it is degraded into the inactive thromboxane B 2 (TXB 2 ) and malonyldialdehyde.
The second pathway of activation and aggregation can proceed completely independently from the first one: various platelet agonists, including thrombin, ADP, TXA 2 and collagen, bind to receptors and via a G-protein mechanism, activate phospholipase C. This generates diacylglycerol and inositol triphosphate, which in turn activate protein kinase C and elevate intracellular calcium, respectively. Calcium is released from the dense tubular system to form complexes with calmodulin. This complex and the free calcium act as coenzymes for the release reaction, for the activation of different regulatory proteins and of actin and myosin and the contractile system and also for the liberation of arachidonic acid from membrane phospholipids and the generation of TXA 2 .
The aggregating platelets join together into loose reversible aggregates, but after the release reaction of the platelet granules, larger, firmer aggregates form. Changes in the platelet membrane configuration now occur; ‘flip-flop’ rearrangement of the surface brings the negatively charged phosphatidyl-serine and -inositol on to the outer leaflet, thus generating platelet factor 3 (procoagulant) activity. At the same time specific receptors for various coagulation factors are exposed on the platelet surface and help coordinate the assembly of the enzymatic complexes of the coagulation system. Local generation of thrombin will then further activate platelets.
The resting endothelium helps maintain platelets in a nonactivated state by secreting prostacyclin and NO. Prostacyclin released locally binds to specific platelet membrane receptors and then activates the membrane-bound adenylate cyclase (producing cyclic adenosine monophosphate, or cAMP). cAMP inhibits platelet aggregation by inhibiting arachidonic acid metabolism and the release of free cytoplasmic calcium ions.
Thus platelets have several roles in haemostasis:
- 1.
Adhesion and aggregation forming the primary haemostatic plug
- 2.
Clot retraction
- 3.
Release of platelet activating and procoagulant molecules
- 4.
Provision of a procoagulant surface for the reactions of the coagulation system.
Blood coagulation
The chief product of the coagulation pathways is thrombin, which cleaves fibrinogen to produce fibrin and thus the fibrin clot. This clot is further strengthened by the crosslinking action of factor XIII (FXIII), which is also activated by thrombin with fibrin acting as a cofactor. The two commonly used coagulation tests, the activated partial thromboplastin time (APTT) and the prothrombin time (PT), have defined two pathways of coagulation activation: the intrinsic and extrinsic paths, respectively. However, this bears only a limited relationship to the way coagulation is activated in vivo. For example, deficiencies of factor XII (FXII) or FVIII both produce marked prolongation of the APTT, but only deficiency of the latter is associated with a haemorrhagic tendency. Moreover, there is considerable evidence that activation of factor IX (FIX) (intrinsic pathway) by activated factor VII (FVIIa) (extrinsic pathway) is crucial to establishing coagulation after an initial stimulus has been provided by FVIIa-tissue factor (TF) activation of factor X (FX). See Figure 18-1 .
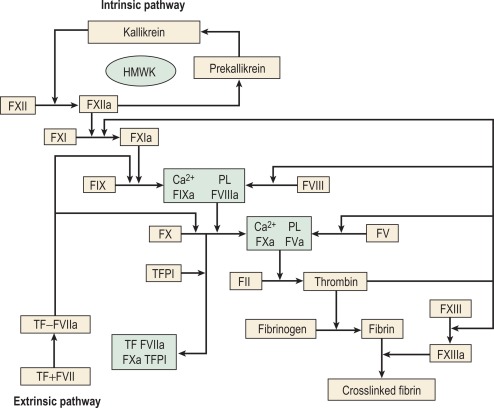
Investigation of the coagulation system centres on the coagulation factors, but the activity of these proteins is also greatly dependent on specific surface receptors and phospholipids largely presented on the surface of platelets and also by activated endothelium. The necessity for calcium in many of these reactions is frequently used to control their activity in vitro. The various factors are described in the following sections, as far as possible in their functional groups; their properties are detailed in Table 18-2 .
| Concentration in Plasma | |||||
|---|---|---|---|---|---|
| No. | Factor | RMM (Daltons) | Half-Life | μg/ml | nmol/l |
| I | Fibrinogen | 340 000 | 90 h | 1.5–4 × 10 6 | − |
| II | Prothrombin | 70 000 | 60 h | 100–150 | 1400 |
| V | − | 330 000 | 12–36 h | 5–10 | 20 |
| VII | − | 48 000 | 6 h | 0.5 | 10 |
| VIII | − | 200 000 | 12 h | 0.2 | 0.7 |
| VWF | − | 800 000–140 000 000 | 10–24 * h | 10 | − |
| IX | − | 57 000 | 24 h | 4 | 90 |
| X | − | 58 000 | 40 h | 10 | 170 |
| XI | − | 158 000 | 60 h | 6 | 30 |
| XII | − | 80 000 | 48–52 h | 30 | 375 |
| Prekallikrein | − | 85 000 | 48 h | 40 | 450 |
| HMWK | − | 120 000 | 6.5 days | 80 | 700 |
| XIII | − | 32 000 | 3–5 days | 30 (A + B) | 900 (tetramer) |
* The half-life of VWF varies according to the ABO blood group being shortest in O, longest in AB and intermediate in A and B.
The contact activation system
The contact activation system comprises FXII (Hageman factor) high molecular weight kininogen (HMWK) (Fitzgerald factor) and prekallikrein/kallikrein (Fletcher factor). As mentioned earlier, these factors are not essential for haemostasis in vivo. Their important activities are to activate the fibrinolytic system, to activate the complement system and to generate vasoactive peptides: in particular, bradykinin is released from HMWK by prekallikrein or FXIIa cleavage. Kallikrein and FXIIa also function as chemoattractants for neutrophils. The contact activation system also has some inhibitory effect on thrombin activation of platelets and prevents cell binding to endothelium. Recent animal evidence implicates the contact system in platelet-dependent thrombosis although a role in humans is not yet established.
When bound to a negatively charged surface in vitro, FXII and prekallikrein are able to reciprocally activate one another by limited proteolysis, but the initiating event is not clear. It may be that a conformational change in FXII on binding results in limited autoactivation that triggers the process. HMWK acts as a (zinc-dependent) cofactor by facilitating the attachment of prekallikrein and factor XI (FXI), with which it circulates in a complex, to the negatively charged surface. There are numerous candidates for the physiological surface on which this takes place, including collagen and polyphosphate. The contact system can also activate fibrinolysis by a number of mechanisms: plasminogen cleavage, urokinase plasminogen activator (uPA) activation and tPA release. Most importantly from the laboratory point of view, the contact activation system generates FXIIa, which is able to activate FXI, thus initiating the coagulation cascade of the intrinsic pathway.
Tissue factor
TF is the cofactor for the extrinsic pathway and the physiological initiator of coagulation. It is a transmembrane protein and constitutively present in many tissues outside the vasculature and on the surface of activated monocytes and, under some conditions, endothelial cells. FVIIa binds to TF in the presence of calcium ions and then becomes enzymatically active. Small amounts of FVIIa are present in the circulation but have virtually no enzymic activity unless bound to TF. The FVIIa-TF complex can activate both FX and FIX and therefore two routes to thrombin production are stimulated. FXa subsequently binds to TFPI and then to FVIIa to form an inactive quaternary (Xa-TF-VIIa-TFPI) complex. This mechanism therefore functions to shut off the extrinsic pathway after an initial stimulus to coagulation has been provided.
The vitamin K-dependent coagulation factors are the serine proteases factors II, VII, IX and X. However the anticoagulant proteins – S, C and Z – are also vitamin K dependent. Each of these proteins contains a number of glutamic acid residues at its amino terminus that are γ-carboxylated by a vitamin K-dependent mechanism. This results in a novel amino acid, γ-carboxyglutamic acid, which by binding calcium promotes a conformational change in the protein allowing it to bind to negatively charged phospholipid. Because this binding is crucial for coordinating the interaction of the various factors, the proteins produced in the absence of vitamin K (PIVKAs) that are not γ carboxylated are essentially functionless. The vitamin K-dependent factors are zymogens which require cleavage, sometimes with release of a small peptide (activation peptide) to become functional. Measurement of these activation peptides has been used as a means of assessing coagulation activation.
Cofactors
Factors VIII and V are the two most labile of the coagulation factors, and they are rapidly lost from stored blood or heated plasma. They share considerable structural homology and are cofactors for the serine proteases FIXa and FXa, respectively. They both require proteolytic activation by factor IIa or Xa to function. FVIII circulates in combination with VWF, which is present in the form of large multimers of a basic 200 kD monomer. One function of VWF is to stabilise FVIII and protect it from degradation. In the absence of VWF, the survival of FVIII in the circulation is extremely short (< 2 h instead of the normal 8–12 h). VWF may also serve to deliver FVIII to platelets adherent to a site of vascular injury. Once FVIII has been cleaved and activated by thrombin it no longer binds to VWF.
Fibrinogen
Fibrinogen is a large dimeric protein, each half consisting of three polypeptides named Aα, Bβ and γ held together by 12 disulphide bonds. The two monomers are joined together by a further three disulphide bonds. A variant γ chain denoted γ′ is produced by a variation in messenger RNA splicing. In the process a platelet binding site is lost and high-affinity binding sites for FXIII and thrombin are gained. The γ′ variant constitutes approximately 10% of plasma fibrinogen. A less common (< 2%) α chain variant ‘γE’ is also produced by splice variation. Fibrinogen is also found in platelets, but the bulk of this is derived not from synthesis by megakaryocytes but from glycoprotein IIbIIIa-mediated endocytosis of plasma fibrinogen, which is then stored in alpha granules. Fibrin is formed from fibrinogen by thrombin cleavage releasing the A and B peptides. This results in fibrin monomers that then associate and precipitate forming a polymer that is the visible clot. The central E domain exposed by thrombin cleavage binds with a complementary region on the outer or D domain of another monomer. The monomers thus assemble into a staggered overlapping two-stranded fibril. More complex interactions subsequently lead to branched and thickened fibre formation making a complex mesh that binds and stabilises the primary platelet plug.
Factor XIII
The initial fibrin clot is held together by noncovalent interactions and can be deformed and resolubilised. FXIII, which is also activated by thrombin, is able to covalently crosslink these fibrin monomers. FXIII is a transglutaminase that joins a glutamine residue on one chain to a lysine on an adjacent chain. This loss of resolubility is the basis of the screening test for FXIII deficiency.
Inhibitors of coagulation
A number of mechanisms exist to ensure that the production of the fibrin clot is limited to the site of injury and the clot is not allowed to propagate indefinitely. , First there are a number of proteins that bind to and inactivate the enzymes of the coagulation cascade. Probably the first of these to become active is TFPI, which rapidly quenches the FVIIa-TF complex that initiates coagulation. It does this by combining first with FXa so that further propagation of coagulation is dependent on the small amount of thrombin that has been generated during initiation being sufficient to activate the intrinsic pathway.
The principal physiological inactivator of thrombin is antithrombin (AT, formerly ATIII), which belongs to the serpin group of proteins. This binds to factor IIa forming an inactive thrombin–antithrombin complex (TAT), which is subsequently cleared from the circulation by the liver. This process is greatly enhanced by the presence of heparin or vessel wall heparan. AT is responsible for approximately 60% of thrombin-inactivating capacity in the plasma; the remainder is provided by heparin cofactor II and less specific inhibitors such as α2 macroglobulin. AT is also capable of inactivating factors X, IX, XI and XII but to lesser degrees than thrombin.
As thrombin diffuses away from the area of damage it binds to thrombomodulin on the surface of endothelial cells. Although remaining available for binding to AT, thrombin bound to thrombomodulin no longer cleaves fibrinogen. It now has a greatly enhanced preference for PC as a substrate. PC is presented to the thrombin–thrombomodulin complex by EPCR and when activated by thrombin cleavage acts to limit and arrest coagulation by inactivating factors Va and VIIIa. This action is further enhanced by its cofactor, protein S, which does not require prior activation. The role of EPCR is particularly important in larger vessels, where the effective concentration of thrombomodulin is low. PC is subsequently inactivated by its own specific inhibitor.
The fibrinolytic system
The deposition of fibrin and its removal are regulated by the fibrinolytic system. Although this is a complex multicomponent system with many activators and inhibitors, it centres on the fibrinogen- and fibrin-cleaving enzyme, plasmin. Plasmin circulates in its inactive precursor form, plasminogen, which is activated by proteolytic cleavage. The principal plasminogen activator (PA) in humans is tissue plasminogen activator, which is another serine protease. tPA and plasminogen are both able to bind to fibrin via the amino acid lysine. Binding to fibrin brings tPA and plasminogen into close proximity so that the rate of plasminogen activation is markedly increased and thus plasmin is generated preferentially at its site of action and not free in plasma. The second important physiological PA in humans is urokinase (uPA). This single-chain molecule (scuPA or pro-urokinase) is activated by plasmin or kallikrein to a two-chain derivative (tcuPA), which is not fibrin-specific in its action. However, the extent to which this is important in vivo is not clear, and the identification of cell surface receptors for uPA suggests that its primary role may be extravascular. The contact activation system also appears to generate some plasminogen activation via FXIIa and bradykinin-stimulated release of tPA. The cleavage products released by the action of plasmin on fibrin are of diagnostic use and are discussed later in this chapter. The activation of plasmin on fibrin is restricted by the action of a carboxypeptidase, which removes the amino terminal lysine residues to which plasminogen and tPA bind. This carboxypeptidase is activated by thrombomodulin-bound thrombin and is referred to as thrombin-activated fibrinolysis inhibitor (TAFI).
Plasminogen activator inhibitor-1 (PAI-1) is a potent inhibitor of tPA, produced by endothelial cells, hepatocytes, platelets and placenta. Levels in plasma are highly variable. It is a member of the serpin family and is active against tPA and tcuPA. A second inhibitor, PAI-2, has also been identified, originally from human placenta, but its role and importance are not yet established.
The main physiological inhibitor of plasmin in plasma is plasmin inhibitor (α2-antiplasmin), which inhibits plasmin function by forming a 1:1 complex (plasmin–antiplasmin complex, PAP). This reaction in free solution is extremely rapid but depends on the availability of free lysine-binding sites on the plasmin. Thus, fibrin-bound plasmin in the clot is not accessible to the inhibitor. Deficiencies of the fibrinolytic system are rare but have sometimes been associated with a tendency to thrombosis or haemorrhage.
General approach to investigation of haemostasis
This section begins with some general points regarding the clinical and laboratory approach to the investigation of haemostasis. Following this, the basic or first-line screening tests of haemostasis are described. These tests are generally used as the first step in investigation of an acutely bleeding patient, a person with a suspected bleeding tendency or as a precaution before an invasive procedure is carried out. They have the virtue that they are easily performed, and the patterns of abnormalities obtained point clearly to the appropriate next set of investigations. However these tests examine only a portion of the haemostatic mechanism and have limited sensitivity for the presence of significant bleeding diatheses such as von Willebrand disease (VWD) or disorders of platelets or vessels. Hence a normal ‘clotting screen’ should not be taken to mean that haemostasis is normal.
Clinical approach
The investigation of a suspected bleeding tendency may begin from three different points:
- 1.
Investigating a clinically suspected bleeding tendency. The investigation properly begins with the bleeding history, which may suggest an acquired or congenital disorder of primary or secondary haemostasis. If the bleeding history or family history is significant, appropriate specific tests and assays should be performed, notwithstanding the results of screening tests such as the PT and APTT. Considerable effort has been put into defining those aspects of clinical history that predict a significant bleeding disorder, and bleeding state questionnaires are now available.
- 2.
Following up an abnormal first-line test. The abnormalities already detected will determine the appropriate further investigations and are described below.
- 3.
Investigation of acute haemostatic failure. This is often required in the context of an acutely ill or postoperative patient. Investigations are therefore directed toward detecting disseminated intra-vascular coagulation (DIC) or a previously undetected coagulation defect (congenital or acquired). The availability of a normal premorbid coagulation screen and further questioning to determine a bleeding history can be extremely useful in this respect.
In all cases, comprehensive clinical evaluation – including the patient’s history, the family history and the family tree, as well as the details of the site, frequency and character of haemorrhagic manifestations – should be considered in conjunction with laboratory results to avoid misinterpretation. The results of the screening investigations, taken in conjunction with clinical information, usually point to the appropriate additional procedure.
Principles of laboratory analysis
As noted above the tests of coagulation performed in the laboratory are imperfect attempts to mimic in vitro processes that normally occur in vivo. More detailed investigations of coagulation proteins also require caution in their interpretation depending on the type of assay performed. These can be divided into four principal categories described below.
Immunological
Immunological tests rely on the recognition of the protein in question by polyclonal or monoclonal antibodies. The antibody is coupled to a system that quantifies the extent of binding. Polyclonal antibodies lack specificity but provide relatively high sensitivity, whereas monoclonal antibodies are highly specific but produce relatively low levels of antigen binding. Immunological assays are often easy to perform, convenient for large batches and can be bought as kits with standardised controls. The obvious drawback of these assays is that they may tell you nothing about the functional capacity of the antigen detected. If possible they should always be carried out in parallel with a functional assay.
The antibody may be bound to a plate but to facilitate automation it is now often bound to microparticles. Antibody binding may then be quantified by some form of luminescence (e.g. chemiluminescence) using a secondary antibody or by changes in turbidity when particles are cross linked (immunoturbidimetric assay). Artefacts in these assays may arise from the presence of rheumatoid factor or other auto-antibodies.
Assays using chromogenic peptide substrates (amidolytic assays)
The serine proteases of the coagulation cascade have narrow substrate specificities and it is possible to synthesise a short peptide specific for each enzyme that has a dye ( p -nitroaniline, p -NA) attached to the terminal amino acid. When the synthetic peptide reacts with the specific enzyme, the dye is released and the rate of its release or the total amount released can be measured photometrically. This gives a measure of the enzyme activity present. Chromogenic substrate assays can be classified into direct and indirect assays. Direct assays can be further subclassified into primary assays, in which a substrate specific for the enzyme to be measured is used, and secondary assays, in which the enzyme or proenzyme measured is used to activate a second protease for which a specific substrate is available. Specific substrates are available for many coagulation enzymes. However, the substrate specificity is not absolute and most kits include inhibitors of other enzymes capable of cleaving the substrate to improve specificity. Indirect assays are used to measure naturally occurring inhibitors and some platelet factors.
The measurement of amidolytic activity is not the same as the measurement of biological activity in a coagulation assay and in some cases may not accurately reflect this. This is particularly important when dealing with the molecular variants of various coagulation factors for which a functional assay may be required. The assays can be automated, carried out in a microtitre plate or in a tube when a spectrophotometer is used to measure the intensity of the colour development.
Coagulation assays
Coagulation assays are functional bioassays and rely on comparison with a control or standard preparation with a known level of activity. In the one-stage system optimal amounts of all the clotting factors are present except the one to be determined, which should be as near to absent as possible. The principles of bioassay, its standardisation and its limitations are considered in detail on page 386.
Coagulation assay techniques are also used in mixing tests to identify a missing factor in an emergency or to identify and quantify an inhibitor or anticoagulant. The advantage of this type of assay is that it most closely approximates the activity in vivo of the factor in question. However, they can be technically difficult to perform and their susceptibility to interference from other plasma components has a detrimental effect on their accuracy and precision.
Fluorescence resonance energy transfer
Fluorescence resonance energy transfer (FRET) describes the distance-dependent transfer of energy from one molecule (the donor) to a second molecule (the acceptor). The transfer of energy leads to a reduction in the donor fluorescence and increase in the acceptor fluorescence. Thus a FRET assay can be used for a function that results in separation of the two molecules. For example, FRET assays for ADAMTS13 involve a chemically modified fragment of the VWF A2 domain spanning the ADAMTS13 cleavage site.
Other assays
Other assays include measurement of coagulation factors using snake venoms, assay of ristocetin cofactor and the amide-release assay for FXIII transglutaminase activity. DNA analysis is sometimes helpful in coagulation and is described in Chapter 8 .
Notes on equipment
Water baths
A 37 °C water bath is required for manual coagulation tests, incubation steps and the rapid thawing of frozen specimens. Water baths should be set at 37 °C and monitored using a certified thermometer to ensure it varies by no more than ± 0.5 °C. Slight variation in temperature will markedly affect the speed of clotting reactions. A water bath with plastic or glass sides is preferable, and cross-illumination helps to determine the exact time of formation and appearance of the fibrin clot. Check that the temperature is 37 °C before and during use. Distilled water should be used to fill the water bath and maintain the water level. Records must be kept.
Refrigerators and freezers
Ensure that the temperature does not move out of the acceptable range of 4° ± 2 °C for refrigerators and − 20° ± 2 °C or − 80 ± 2 °C (as applicable) for freezers, rechecking during the day. Records must be kept. Freezers should maintain a constant temperature and so should not auto-defrost.
Centrifuges
Check to ensure each machine is clean before and after use. Also do a visual inspection of rotors, buckets and liners for corrosion and cracks. Thorough maintenance records should be kept.
Reagents and buffers
Attention must be paid to the age and condition of solutions. This is particularly important with the calcium chloride solution. Whenever a solution is prepared it should be correctly labelled and dated. Buffers should be inspected for bacterial growth before use: contamination with microorganisms can cause errors and assay failures as a result of the release of enzymes and other active biological substances into solution. Azide may be added as a preservative to some buffers but should not be used in reagents for platelet studies or enzyme-linked immunosorbent assay (ELISA) substrates. Chromogenic substrates should be reconstituted with sterile distilled water; contamination with bacterial enzymes may cause pNA release and yellow discolouration of the reagent. Records of batch numbers, in use dates and expiry dates should be kept.
Plastic and glass tubes
For clotting tests, 75 × 10 mm glass rimless test tubes should be used. Plastic tubes should be used for sample dilutions, storage and reagent preparation.
Pipettes
A range of graduated glass (certified Class A) and automatic pipettes must be obtained. The latter should be accurate and durable. Fluids should not be drawn into the pipette barrels and acids should not be pipetted with instruments containing metal piston assemblies, which may become pitted or corroded. Attention to technique is vital because contamination of reagents with used pipette tips may occur, there may be errors of volume as a result of fluid on the exterior of the pipette tip, or the manner of addition of a reagent may alter the results obtained. The amount of fluid drawn into the tip should be inspected visually with each pipetting procedure. Records of pipette accuracy and precision should be kept.
Stopwatches and clocks
Stopclocks are useful for timing incubation periods of several minutes or more, but stopwatches that can be held in the hand and controlled rapidly should be used for measuring clotting times and for short incubations. At least four stopwatches are needed unless an automated coagulometer is used.
Automated coagulation analysers
A wide variety of automated and semi-automated coagulation analysers are available. The choice of analyser depends on predicted workload, repertoire and cost implications. Evaluation of laboratory equipment for coagulation testing is detailed in previous editions of this book and now in Chapter 24 .
Pre-analytical variables including sample collection
Many misleading results in blood coagulation arise not from errors in testing but from carelessness in the pre-analytical phase.
When blood is withdrawn from a vessel, changes begin to take place in the components of blood coagulation. Some occur almost immediately, such as platelet activation and the initiation of the clotting mechanism dependent on surface contact.
It is essential to take precautions at this early stage to prevent, or at least minimise, in vitro changes by conforming to recommended criteria during collection and storage. These criteria, as described below, have been established by the Clinical and Laboratory Standards Institute (CLSI).
Collection of venous blood
Venous blood samples should be obtained whenever possible, even from the neonate. Capillary blood tests require modification of techniques, experienced operators and locally established normal ranges; they are not an easy alternative to tests on venous blood. Patients requiring venepuncture should be relaxed and in warm surroundings. Excessive stress and vigorous exercise cause changes in blood clotting and fibrinolysis. Stress and exercise will increase FVIII, VWF and fibrinolysis.
Whenever possible, venous samples should be collected without a pressure cuff, allowing the blood to enter the syringe by continuous free flow or by the negative pressure from an evacuated tube (see p. 2). Venous occlusion causes haemoconcentration, increase of fibrinolytic activity, platelet release reaction and activation of some clotting factors. In the majority of patients, however, light pressure using a tourniquet is required; this should be applied for the shortest possible time (e.g. less than 1 min). The venepuncture must be ‘clean’; blood samples from an indwelling line or catheter should not be used for tests of haemostasis because they are prone to dilution and heparin contamination. If there is no other source of blood then the line must be flushed thoroughly before sampling and the results scrutinised for possible artefacts.
To minimise the effects of contact activation, good-quality plastic or polypropylene syringes should be used. If glass blood containers are used, they should be evenly and adequately coated with silicone.
The blood is thoroughly mixed with the anticoagulant by inverting the container several times. The samples should be brought to the laboratory as soon as possible. If urgent fibrinolysis tests are contemplated, the blood samples should be kept on crushed ice until delivered to the laboratory. Assays of tPA and of PA1-1 antigen are preferably performed on samples taken into trisodium citrate to prevent continued tPA-PA1-1 binding (see p. 562).
If an evacuated tube system is used for collecting samples for different tests, the coagulation sample should be the second or third tube obtained.
Blood sample anticoagulation
The most commonly used anticoagulant for coagulation samples is trisodium citrate. A 32 g/1 (0.109 M) solution (p. 562) is recommended. Other anticoagulants, including oxalate, heparin and ethylenediaminetetra-acetic acid (EDTA) are unacceptable. The labile factors (factors V and VIII) are unstable in oxalate, whereas heparin and EDTA directly inhibit the coagulation process and interfere with end-point determinations. Additional benefits of trisodium citrate are that the calcium ion is neutralised more rapidly in citrate, and APTT tests are more sensitive to the presence of heparin.
For routine blood coagulation testing, 9 volumes of blood are added to 1 volume of anticoagulant (i.e. 0.5 ml of anticoagulant for a 5 ml specimen). When the haematocrit is abnormal due to either severe anaemia or polycythaemia, the blood:citrate ratio should be adjusted. For a 5 ml specimen (total), the amount of citrate should be as follows:
| Haematocrit | Citrate (ml) |
|---|---|
| 0.20 | 0.70 |
| 0.25 | 0.65 |
| 0.30 | 0.61 |
| 0.55 | 0.39 |
| 0.60 | 0.35 |
| 0.65 | 0.30 |
| 0.70 | 0.26 |
Time of sample collection
The time of day when the sample is collected can be an important factor in the interpretation of results. Fibrinolytic activity follows a definite circadian pattern with a trough at around 6 a.m.
The timing of the collection of the blood sample in relation to drug administration should also be taken into consideration (e.g. after subcutaneous heparin therapy, direct acting anticoagulants and desmopressin).
The timing following administration of factor concentrate samples is very important. The following times are recommended.
FVIII: at 15 min
FIX: at 30 min
Transportation to the laboratory
An efficient and regular collection service is necessary. It is important that samples are delivered as quickly as possible to prevent deterioration of the labile clotting factors such as factors V and VIII. Automated systems can facilitate rapid delivery, but should be avoided when platelet function tests are to be performed because the associated trauma may affect results.
Centrifugation: preparation of platelet-poor plasma
Most routine coagulation investigations are performed on platelet-poor plasma (PPP), which is prepared by centrifugation at 2000 g for 15 min at 4 °C (approximately 4000 rev/min in a standard bench cooling centrifuge). The sample should be kept at room temperature if it is to be used for PT tests, lupus anticoagulant (LAC) or factor VII assays and it should be kept at 4 °C for other assays. The testing should preferably be completed within 2 h of collection. Care must be taken not to disturb the buffy coat layer when removing the PPP.
Samples for platelet function testing, LAC and the activated PC resistance (APCR) test should not be centrifuged at 4 °C. These samples should be prepared by centrifugation at room temperature to prevent activation of platelets and release of platelet contents such as phospholipid and factor V. For LAC testing and APCR it is very important that the number of platelets and the amount of platelet debris in the samples is minimised. The platelet count should be below 10 × 10 9 /l. This is best achieved by double centrifugation. Filtration of the plasma through a 0.2 μm filter is not recommended because it may create microparticles.
Storage of plasma and sample thawing
Some tests such as the PT and APTT are carried out on fresh samples. Certain coagulation assays, unless urgently required, can be performed in batches at a later date on deep frozen plasma. Storage of small aliquots of samples in liquid nitrogen (− 196 °C) is the optimum, although samples may be frozen at − 40 °C or − 80 °C for several weeks without significant loss of most haemostatic activities. Gentle but thorough mixing of samples is essential after thawing and before testing. Once thawed the sample should never be refrozen. Samples should be thawed at 37 °C to avoid forming cryoprecipitate.
Some common ‘technical’ errors
A false abnormality of the clotting time may occur in the following situations:
- 1.
Faulty collection of the sample, resulting in it undergoing partial clotting
- 2.
Underfilling or overfilling of the bottle or high or low haematocrit causing an incorrect volume of citrate in relation to the volume of plasma
- 3.
An unsuitable anticoagulant, such as EDTA, used in collecting the sample
- 4.
Collection of blood through a line that has been in contact with heparin or used for concentrate infusion
- 5.
Contamination of the kaolin/platelet substitute reagent with a trace of thromboplastin
- 6.
Delay in sample analysis
- 7.
Use of inaccurate pipettes (documentation of pipette calibration is essential)
- 8.
Machine malfunction
- 9.
Incorrect water bath temperature
- 10.
Calcium chloride at incorrect concentration or not freshly prepared.
Calibration and quality control
Reference standard (calibrator)
International (World Health Organisation, WHO) and national standards are available for a number of coagulation factors (see p. 536). For diagnostic tests it is necessary to test a calibrated normal reference preparation alongside the patients’ plasmas.
Because the concentration of some coagulation factors may vary as much as fourfold in different normal plasma samples, it is inadvisable to use plasma from any one person to represent 100% clotting activity. It is recommended that a calibrated reference plasma be routinely used with each assay. If this is not possible, then a locally prepared normal pool can be used, provided it is itself calibrated against a reference preparation. If it is necessary to use an uncalibrated plasma pool then the larger the number of donors, the closer the pool clotting activity will be to 100% or 1 iu/ml. A suggested minimum for a normal pool is 20 donors.
Calibration of standard pools and suggested calibration procedure
Whenever possible, the normal pool should be calibrated against a reference material already calibrated against the international standard. The reference material may be a national standard (e.g. National Institute for Biological Standards and Control, www.nibsc.org ) or a commercial standard. In the absence of reference materials, the laboratory should obtain as large a normal pool as possible and assign it a value of 1 iu/ml.
The most important principle of calibration is repetition to minimise possible errors at each stage. It is necessary to carry out at least four independent assays and preferably six. An independent assay is an assay for which a new ampoule of standard is opened, or if a freeze-dried standard is not available, for which a new set of dilutions are prepared from frozen previous reference plasma. Each plasma must be tested in duplicate; two replicate assays should be carried out each day and the procedure should be repeated on at least 4 days (four independent assays). Whenever possible more than one operator should be involved.
Comparison should always be made with the previous normal pool. The potency of the new normal pool is calculated for each replicate assay on each day and an overall mean value is calculated. This calibration also enables an assessment of the precision of the method used.
Control plasma
Controls are included alongside patient samples in a batch of tests. Inclusion of both normal and abnormal controls will enable detection of nonlinearity in the standard curve. Whereas a reference standard (calibrator) is used for accuracy, controls are used for precision. Precision control, the recording of the day-to-day variation in control values, is an important procedure in laboratory coagulation. Participation in an external assessment scheme (see p. 539) is also important to ensure inter-laboratory harmonisation. The use of lyophilised reference standard and control plasmas has become widespread, whereas locally calibrated standard pools are used especially in under-resourced laboratories. The results of participation in external quality-control schemes require careful attention. The large number of different reagents, substrate plasmas, reference preparations and analysers available makes comparison of like with like difficult. Ideally all combinations should give similar results, but this is often not the case and the results should be used to carefully choose the combination to be used.
A control must be stable and homogeneous; the exact potency is not important, although the approximate value should be known to select preparations at the upper and lower limit of the normal reference range. Third party controls with assigned values should be used.
Fresh control blood is required for procedures such as platelet aggregation and should be obtained from ‘normal’ healthy subjects. Fresh controls should be prepared in exactly the same way as the patient sample.
Variability of coagulation assays
Within a laboratory, variability is most commonly the result of a dilution error, differences in the composition of reagents, failure to take the time trend into account and differences in experience and technique between operators.
Variability between laboratories is much higher. Apart from the factors described for the within-laboratory variability, there is the major effect of differences in methods and in the composition of reagents. Comparability between laboratories improves if standardised reagents and techniques are used.
The unavoidable variability associated with coagulation assays makes the use of reliable reference materials imperative.
Performance of coagulation tests
Handling of samples and reagents
All plasma samples should be kept in plastic or siliconised glass tubes and placed on melting ice or at 4 °C until used, except when cold activation of factor VII and platelets is to be avoided, in which case the plasma is kept at room temperature. All pipetting should be performed using disposable plastic pipettes or autodiluter pipette tips. Manual clotting tests are performed at 37 °C in new round-bottom glass tubes of standard size (10 or 12 mm external diameter). Ideally, all glassware should be disposable. If the tubes have to be reused, scrupulous cleaning using chromic acid and a detergent such as 2% Decon 90 (Decon Laboratories Ltd, www.decon.co.uk ) is essential.
Eliminating a time trend
The potential instability of biological reagents used in tests of haemostasis makes it desirable to arrange results so as to reduce time-related errors. Thus, if there is a significant length of time between the test with the patient’s plasma and the test with the control sample, any difference may be the result of the deterioration of one or more of the reagents or of the plasma itself rather than a true defect or deficiency. In the simplest case, if there are two samples A and B, the readings should be carried out in the order A 1 , B 1 , B 2 , A 2 . Additional specimens are allowed for by inserting further letters into the design.
Assay monitoring and end-point detection
Manual methods
Detecting clot formation as the end-point depends to some extent on the rate of its formation: the shorter the clotting time the more opaque is the clot and the easier it is to detect. A slowly forming clot may appear as mere fibrin wisps, which are difficult to detect by eye or machine. In manual work, the observer must try to adopt a uniform convention in selecting the moment in clot formation that will be accepted as the end-point. It is also important to ensure that the tube can be watched with its lower part under the water or while being quickly dipped in and out so as to avoid cooling and a slowing down of the clot formation. Bubbles also make the determination of the end-point difficult.
Manual clotting techniques are still used in WHO calibration schemes and therefore should be viewed as an essential skill despite the ever-increasing reliance on automation. End-point detection by analysers sometimes fails and dubious or inconsistent results or samples which fail to record a clotting time should be checked manually.
In instrumental work, the coagulometer must be shown to detect long clotting times reliably and reproducibly. The various coagulometers available have different means of detecting the end-point, which may make comparison of results difficult. Some commonly used techniques follow.
Electromechanical
Impedance, steel ball, rotating cuvette
The sample cuvette rotates and a steel ball remains stationary in a magnetic field until the formation of fibrin strands around the ball produces movement (e.g. Amelung KC4, www.tcoag.com ). This is detected by a change in the magnetic field and the coagulation time is recorded.
Impedance, steel ball, rotating steel ball
A steel ball rotates under the influence of a magnet until the formation of fibrin strands around the ball stops it rotating (e.g. Axis Shield Group Thrombotrack www.axis-shield.com ). This is detected by a sensor and the coagulation time is recorded.
Photo-optical analysis
Scattered light detection for clotting assays
The increasing turbidity during the formation of a fibrin clot is measured as an increase in scattered light intensity when exposed to light at a wavelength of 660 nm.
Transmitted light detection for chromogenic assays (405 nm, 575 nm, 800 nm)
Colour production leads to a change in light absorbance, which is detected as a change in transmitted light. The change in absorbance per minute is calculated (Δ optical density (OD)/min). Various wavelengths can be used, such as 405 nm, 575 nm and 800 nm.
Transmitted light detection for immunoassays (405 nm, 575 nm, 800 nm)
The change in light absorbance caused by the antigen–antibody reaction is detected as the change in transmitted light. The change in absorbance per minute is calculated (Δ OD/min). Some analysers detect light transmittance at multiple wavelengths between 395 and 710 nm.
Nephelometry (IL ACL analysers)
Nephelometry is the determination of the intensity of light scatter using a detector placed at right angles to the incident light path and detecting light of the same wavelength as the incident light. The technique is particularly useful in measuring complexes of antigen and antibody produced by immunoprecipitation.
Photo-optical end point determination and analyses
Using the techniques above, a number of methods can be used to define the change in optical transmission that corresponds to the endpoint of the reaction.
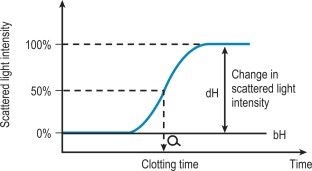
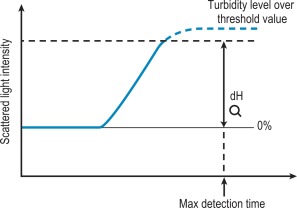
Percentage detection method
After initiating the clotting reaction, the transmitted light is monitored and a baseline value (bH) is determined for the reaction (bH = 0%) ( Fig. 18-2 ). The reaction is then monitored until the clotting reaction is completed (dH = 100%). The time to an arbitrarily set end point, usually 50%, is then determined. At this point the H value usually shows the greatest rate of change and the fibrin monomer polymerisation reaction rate is high. Detection based on this principle enables coagulation analysis to be more accurate at low fibrinogen concentrations in samples with low scatter and those samples for which the initial scatter is higher than usual, such as lipaemic and haemolysed samples.
Rate method
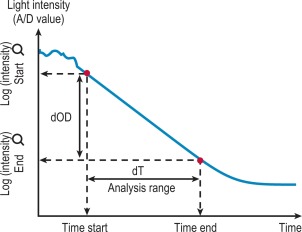
After the start of the reaction the change in absorbance is monitored ( Fig. 18-3 ). After a predetermined time the final absorbance measurement is made. The rate of the absorbance increase per minute between these two time point measurements is calculated. The calculated rate of change in absorbance (Δ OD/min) is used to construct a standard curve (i.e. there is no end-point per se ). This method is used for activity measurement such as in chromogenic substrate assays. It is important that the rate is measured on a linear portion of the curve.
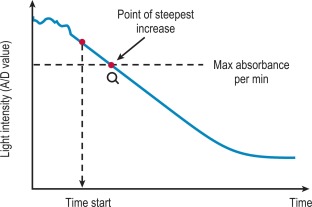
VLin integral method
The VLin integral method evaluates the absorbance per minute of an immunological reaction ( Fig. 18-4 ). This is monitored and mathematical analysis is used to determine the peak rate of change (maximum velocity). The VLin integral evaluation method is used for immunological assays including those for D-dimer and VWF antigen. Using this method allows increased sensitivity, extended measuring range, reduced measurement time and improved antigen excess reliability when measuring an immunological reaction.
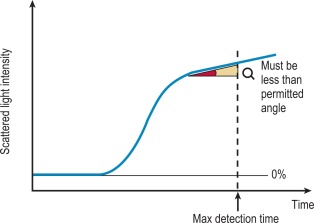
Analysis time over
The ‘Analysis Time Over’ check is used to detect whether the reaction end-point is correct and an endpoint has been reached ( Fig. 18-5 ). If the sample reaction end angle is greater than the permitted angle at the maximum detection time, the result will be flagged with an ‘Analysis Time Over’ error. The situation occurs when testing samples with prolonged clotting times and a satisfactory end point has not been reached by the end of the time allotted for analysis. When this occurs the following checks should be performed:
- 1.
Check the sample for possible anticoagulant contamination, haemolysis, lipaemia, hyperbilirubinaemia or turbidity.
- 2.
Verify delivery of sample and reagent.
- 3.
Set the ‘Maximum Reading Time’ to a longer time and reanalyse the sample.
- 4.
If reanalysis of the sample results in a numerical value without an error flag, the result can be reported.
- 5.
If reanalysis gives an ‘Analysis Time Over’ message again, the sample may not be capable of forming a firm clot. In these situations the clotting time must be checked manually.
Turbidity level over
If the dH exceeds the detection capacity of the analogue to digital converter, the result will not be reported and it may be suspected that the sample plasma is turbid or lipaemic ( Fig. 18-6 ). When this occurs the following checks should be performed:
- 1.
Check the sample for turbidity, lipaemia, haemolysis or hyperbilirubinaemia.
- 2.
Verify delivery of sample and reagent.
- 3.
For a fibrinogen assay, dilute the sample with Owren veronal buffer and reanalyse.
- 4.
If reanalysis of the sample results in a numerical value without an error flag, the result can be reported.
- 5.
Clotting tests such as PT, APTT and thrombin time (TT) must be performed manually.
Clot signatures: normal and abnormal activated partial thromboplastin time clot waveforms
On some analysers the clot waveform can be generated from the monitoring of light transmission or absorbance during the PT or APTT ( Fig. 18-7 ). This has been used to assess procoagulant activity and to detect DIC but is not in routine practice.
Commonly used reagents
Some reagents are common to the majority of first-line tests. They are described here, whereas the reagents specific for one particular test or assay are described with the relevant test.
CaCl 2
The working solution is best prepared from a commercial molar solution. Small volumes of 0.025 mol/l concentration should be prepared frequently and stored for short periods to avoid proliferation of microorganisms. Prewarmed CaCl 2 should always be discarded at the end of the working day. Commercial solutions are available.
Barbitone buffer
- •
50 ml sodium diethyl barbiturate (C 8 H 11 O 3 N 2 Na) 0.2 M (41.2 g/l)
- •
Add 32.5 ml hydrochloric acid (HCl) 0.2M
- •
Make up to 200 ml with water (pharmacy grade distilled water www.baxterhealthcare.co.uk ) and correct pH to 7.4 with HCl.
Barbitone buffered saline, pH 7.4
- •
NaCl 5.67 g
- •
Barbitone buffer, pH 7.4, 1 litre
- •
Before use, dilute with an equal volume of 9 g/l NaCl.
pH 7.3–7.4 is recommended for most clotting tests.
Glyoxaline buffer.
Dissolve 2.72 g of glyoxaline (imidazole) and 4.68 g of NaCl in 650 ml of water. Add 148.8 ml of 0.1 mol/l HCl and adjust the pH to 7.4. Adjust the volume to 1 litre with water.
Owren veronal buffer
- •
Sodium acetate: 3.89 g
- •
Barbitone sodium: 5.89 g
- •
Sodium chloride: 6.8 g
- •
Dissolve the salts in 800 ml of water.
- •
Add 21.5 ml of 1 mol/l HCl, then make up to 1 litre with water, mix and check that the pH is 7.4.
Alternatively the solutions can be purchased commercially ( www.sigmaaldrich.com ).
Factor-deficient plasmas
Plasmas deficient in specific factors are required for many bioassays. They may be obtained from individuals with congenital deficiency of the factor, but frequently these patients will have been treated with plasma concentrates and there is a danger of infection. Many laboratories now use commercial plasmas rendered deficient in the factor by immunodepletion and then lyophilised. However, it is important to establish that these are completely deficient. Once reconstituted, lyophilised plasmas should be gently mixed and left to stand for 20 min before use. If an automated coagulation analyser is used, the factor-deficient plasma should be placed in position 10 min prior to testing.
The ‘clotting screen’
Basic tests of coagulation are often performed with no specific diagnosis in mind and in the absence of any clinical indication of a haemostatic disorder. There may be numerous reasons for this and the tests performed may give clues to diagnosis or may detect an unsuspected hazard that increases the risk of postoperative bleeding. Equally, they may produce false-positive abnormalities that cause concern and confusion and delay procedures. The choice and extent of tests performed in this screening process will vary between hospitals.
Prothrombin time
Principle
The prothrombin time measures the clotting time of recalcified plasma in the presence of an optimal concentration of tissue extract (thromboplastin) and indicates the overall efficiency of the extrinsic clotting system. Although originally thought to measure prothrombin, the test also depends on factors V, VII and X and on the fibrinogen concentration of the plasma.
Reagents
Patient and control plasma samples
Platelet-poor plasma from the patient and control is obtained as described on p. 375. Note that plasma stored at 4 °C may have a shortened PT as a result of factor VII activation in the cold.
Thromboplastin
Thromboplastins were originally tissue extracts obtained from different species and different organs containing tissue factor and phospholipid. Because of the potential hazard of viral and other infections from handling human brain, it should no longer be used as a source of thromboplastin. A laboratory method for a rabbit brain preparation, of use in under-resourced laboratories, is described on page 556 and in previous editions.
Recombinant thromboplastins are manufactured using recombinant human tissue factor produced in Escherichia coli and synthetic phospholipids, which do not contain any other clotting factors. Therefore they are highly sensitive to factor deficiencies and oral anticoagulant-treated patient plasma samples and have an International Sensitivity Index (ISI) close to 1.
Each preparation has a different sensitivity to clotting factor deficiencies and defects, in particular the defect induced by oral anticoagulants. For control of oral anticoagulation a preparation calibrated against the International Reference Thromboplastin should be used (see Chapter 20 ).
CaCl 2
0.025 mol/l.
Method
Deliver 0.1 ml of plasma into a glass tube placed in a water bath and add 0.1 ml of thromboplastin. Wait 1–3 min to allow the mixture to warm. Then add 0.1 ml of warmed CaCl 2 and start the stopwatch. Mix the contents of the tube and record the end-point. Carry out the test in duplicate on the patient’s plasma and the control plasma. When a number of samples are to be tested as a batch, the samples and controls must be suitably staggered to eliminate time bias. Some thromboplastins contain calcium chloride, in which case 0.2 ml of thromboplastin-Ca is added to 0.1 ml plasma and timing is started immediately.
Expression of results
The results are expressed as the mean of the duplicate readings in seconds or as the ratio of the mean patient’s plasma time to the mean normal control plasma time. The control plasma is obtained from 20 normal men and women (the latter not pregnant and not taking oral contraceptives) and the logarithmic mean normal PT (LMNPT) is calculated. For further details and a discussion of the importance of the PT in oral anticoagulant control, when results may be reported as an International Normalised Ratio (INR), see Chapter 20 .
Normal values
Normal values depend on the thromboplastin used, the exact technique and whether visual or instrumental end-point reading is used. With most rabbit thromboplastins the normal range of the PT is between 11 and 16 sec; for recombinant human thromboplastin it is somewhat shorter (10–12 sec). Each laboratory should establish its own normal range.
Interpretation
The common causes of a prolonged PT are as follows:
- 1.
Administration of oral anticoagulant drugs (vitamin K antagonists)
- 2.
The presence of a direct acting inhibitor of factor Xa
- 3.
Liver disease, particularly obstructive jaundice
- 4.
Vitamin K deficiency
- 5.
Disseminated intravascular coagulation
- 6.
Rarely, a previously undiagnosed factor VII, X, V or prothrombin deficiency or defect.
Note: With prothrombin, FX or factor V deficiency the APTT will also be prolonged.
Activated partial thromboplastin time
Specific variations of the APTT test are known as the partial thromboplastin time with kaolin (PTTK) and the kaolin cephalin clotting time (KCCT), reflecting the methods used to perform the test.
Principle
The test measures the clotting time of plasma after the activation of contact factors and the addition of phospholipid and CaCl 2 but without added tissue thromboplastin and so indicates the overall efficiency of the intrinsic pathway. The plasma is first preincubated for a set period with a contact activator such as kaolin, silica or ellagic acid. During this phase of the test, FXIIa is produced, which cleaves FXI to FXIa, but coagulation does not proceed beyond this point in the absence of calcium. After recalcification, FXIa activates FIX and coagulation follows. A standardised phospholipid is provided to allow the test to be performed on PPP. The test depends not only on the contact factors and on factors VIII and IX, but also on the reactions with factors X, V, prothrombin and fibrinogen. It is also sensitive to the presence of circulating anticoagulants (inhibitors) and heparin.
Reagents
PPP
From the patient and a control, stored as described on p. 376.
Kaolin
5 g/l (laboratory grade) in barbitone buffered saline, pH 7.4 (p. 380). Add a few glass beads to aid resuspension. The suspension is stable at room temperature. Other insoluble surface active substances such as silica, celite or ellagic acid can also be used.
Phospholipid
Many reagents are available; these contain different phospholipids.
When choosing a reagent for the APTT, it is important to establish that the activator–phospholipid-incubation time combination is sensitive to deficiencies of factors VIII, IX and XI at concentrations of 0.35–0.4 iu/ml. Combinations that do not produce a prolonged clotting time at these levels are too insensitive. The system should also be responsive to unfractionated heparin over the therapeutic range of approximately 0.3–0.7 anti-Xa iu/ml. In addition, some laboratories will wish the system to be sensitive to the presence of lupus anticoagulants.
CaCl 2
0.025 mol/l.
Method
Mix equal volumes of the phospholipid reagent and the kaolin suspension and leave in a glass tube in the water bath at 37 °C. Place 0.1 ml of plasma into a second glass tube. Add 0.2 ml of the kaolin–phospholipid solution to the plasma, mix the contents and start the stopwatch simultaneously. Leave at 37 °C for 10 min with occasional shaking. At exactly 10 min add 0.1 ml of prewarmed CaCl 2 and start a second stopwatch. Record the time taken for the mixture to clot. Repeat the test at least once on both the patient’s plasma and the control plasma. It is possible to do four tests at 2-min intervals if sufficient stopwatches are available.
Expression of results
Express the results as the mean of the paired clotting times.
Normal range
The normal range is typically 26 to 40 s. The actual times depend on the reagents used and the duration of the preincubation period, which varies in manufacturers’ recommendations for different reagents. Laboratories can choose appropriate conditions to achieve the sensitivity they require. Each laboratory should calculate its own normal range.
Interpretation
The common causes of a prolonged APTT are as follows:
- 1.
Disseminated intravascular coagulation
- 2.
Liver disease
- 3.
Massive transfusion with plasma-depleted red blood cells
- 4.
Administration of or contamination with heparin or other anticoagulants
- 5.
A nonspecific circulating anticoagulant (such as an LAC)
- 6.
The presence of a direct acting anticoagulant drug (e.g. anti-IIa or anti-Xa agents)
- 7.
Deficiency of a coagulation factor other than factor VII
The APTT is also moderately prolonged in patients taking oral anticoagulant drugs and in the presence of vitamin K deficiency. Occasionally, a patient with previously undiagnosed haemophilia or another congenital coagulation disorder presents with an isolated prolonged APTT. If the patient’s APTT is abnormally long, mixing tests, an inhibitor screen and factor assays should be considered (see below).
Thrombin time
Principle
Thrombin is added to plasma and the clotting time is measured. The TT is affected by the concentration and function of fibrinogen and by the presence of inhibitory substances. The clotting time and the appearance of the clot are both informative.
Reagents
PPP
From the patient and a control.
Thrombin solution
A commercial bovine thrombin is used. It is stored frozen as a 50 National Institutes of Health (NIH) unit solution, and it is freshly diluted in barbitone buffered saline in a plastic tube so as to give a clotting time of normal plasma of 15 s (usually approximately 7–8 NIH thrombin units per ml). Shorter times with normal plasma may fail to detect mild abnormalities.
Method
Add 100 μl thrombin solution to 200 μl of control plasma in a glass tube at 37 °C and start the stopwatch. Measure the clotting time and observe the nature of the clot (e.g. whether transparent or opaque, firm or wispy). Repeat the procedure with two tubes containing the patient’s plasma in duplicate and then with a second sample of control plasma.
Expression of results
The results are expressed as the mean of the duplicate clotting times in seconds for the control and the test plasma.
Normal range
A patient’s TT should be within 2 s of the control (i.e. 15 to 19 s). Times of 20 s and longer are definitely abnormal.
Interpretation of results
The common causes of prolonged TT are as follows:
- 1.
Hypofibrinogenaemia as found in DIC and, more rarely, in a congenital deficiency
- 2.
Dysfibrinogenaemia, either inherited or acquired, in liver disease or in neonates
- 3.
Extreme prolongation of the TT is nearly always a result of thrombin inhibition; typically unfractionated heparin but also oral or parenteral direct thrombin inhibitors. If a thrombin inhibitor is suspected, a reptilase time test can be carried out (see p. 386) or the test can be repeated after the addition of heparinase. Low molecular weight heparin (LMWH) produces only a slight prolongation at therapeutic levels
- 4.
Raised concentrations of fibrin degradation products (FDP), as encountered in DIC or liver disease
- 5.
Hypoalbuminaemia
- 6.
Paraproteinaemia.
Shortening of the TT occurs in conditions of coagulation activation.
A transparent bulky clot is found if fibrin polymerization is abnormal, as is the case in liver disease and some congenital dysfibrinogenaemias.
A gross elevation of the plasma fibrinogen concentration may also prolong the TT. Correction can be obtained by diluting the patient’s plasma with saline (see p. 386).
Measurement of fibrinogen concentration
Numerous methods of determining fibrinogen concentration have been devised including clotting, immunological, physical and nephelometric techniques, and all tend to give slightly different results, presumably because of the heterogeneous nature of plasma fibrinogen. Automated analysers can estimate fibrinogen concentration from the coagulation changes during the PT (PT-derived fibrinogen). This is simple, inexpensive and widely used but is not recommended because it is inaccurate (overestimates fibrinogen) in some disease states and in patients who are anticoagulated. Guidelines on fibrinogen assays have been published and recommend the Clauss technique for routine laboratory use.
Fibrinogen assay (Clauss technique)
Principle
Diluted plasma is clotted with a strong thrombin solution; the plasma must be diluted to give a low level of any inhibitors (e.g. FDPs and heparin). A strong thrombin solution must be used so that the clotting time over a wide range is independent of the thrombin concentration.
Reagents
- •
Calibration plasma. With a known level of fibrinogen calibrated against an International Reference Standard
- •
PPP. From the patient and a control
- •
Thrombin solution. Freshly reconstituted to 100 NIH u per ml in 9 g/l NaCl
- •
Owren veronal buffer, pH 7.4. See p. 380.
Method
A calibration curve is prepared each time the batch of thrombin reagent is changed or there is a drift in control results; this is used to calculate the results of unknown plasma samples.
Make dilutions of the calibration plasma in veronal buffer to give a range of fibrinogen concentrations (1 in 5, 1 in 10, 1 in 20 and 1 in 40). Part (0.2 ml) of each dilution is warmed to 37 °C, 0.1 ml of thrombin solution is added and the clotting time is measured. Each test should be performed in duplicate. Plot the clotting time in seconds against the fibrinogen concentration in g/l on log/log graph paper. The 1 in 10 concentration is considered to be 100% and there should be a straight line connection between clotting times of 5 and 50 s. Make a 1 in 10 dilution of each patient’s sample and clot 0.2 ml of the dilution with 0.1 ml of thrombin.
The fibrinogen level can be read directly off the graph if the clotting time is between 5 and 50 s. However, outside this time range a different assay dilution and arithmetical correction of the result will be required (i.e. if the fibrinogen level is low and a 1 in 5 dilution is required, divide answer by 2; for a 1 in 20 dilution multiply answer by 2).
The clot formed in this method may be ‘wispy’ as a result of the plasma being diluted and end-point detection may be easier with optical or mechanical automated equipment. These have been assessed with available substrates and give reasonably consistent results. The high concentration of thrombin used raises the risk of carry over into subsequent tests.
Normal range
The normal range is approximately 1.8 to 3.6 g/l.
Interpretation
The Clauss fibrinogen assay is usually low in inherited dysfibrinogenaemia but is insensitive to heparin unless the level is very high (> 0.8 u/ml). High levels of FDPs (> 190 μg/ml), may also interfere with the assay. Because the chronometric Clauss assay is a functional assay it will generally give a relevant indication of fibrinogen function in plasma. When an inherited disorder of fibrinogen is suspected, a physicochemical estimation should be obtained (e.g. clot weight estimate of fibrinogen or total clottable fibrinogen or an immunological assay; see page 392). If a dysfibrinogenaemia is present, it will reveal a discrepancy between the (functional) Clauss assay and the physical amount of fibrinogen present.
Platelet count
Before considering further investigation of a suspected bleeding disorder always check the platelet count and the blood film (for size and staining characteristics of platelets).
Interpretation of first-line tests
The pattern of abnormalities obtained using the first-line tests described earlier often gives an indication of the underlying defect and determines the appropriate further tests required to define it. The patterns are outlined in Table 18-3 with suggestions for further testing. The choice of second-line investigation will be determined partly by the pattern and degree of abnormality detected in the screening tests but also by the clinical circumstance and history, including use of anticoagulant therapy. Unfortunately it has become apparent that significant anticoagulant effects from LMWH and the direct acting inhibitors of Xa and thrombin may be accompanied by little or no abnormality of the screening tests in some circumstances. When drug ingestion has not been excluded, specific assays may be necessary to exclude their presence.