The term invasive breast carcinoma refers to a heterogeneous group of epithelial malignancies that originate in breast tissue and are characterized by their ability to invade adjacent normal tissue, and metastasize to body sites distant to the breast. The vast majority of these tumors are adenocarcinomas, and they are thought to arise from mammary epithelial cells in the terminal ductulo lobular unit (TDLU) of the breast.
The traditional histologic classification of invasive breast carcinoma provides prognostic and predictive information about the biological behavior of a particular tumor that supplements the other major prognostic indicators such as lymph node status, histologic tumor grade, tumor size, and lymphovascular invasion. In the future, it is likely that new technologies that allow for the rapid genetic and expression profiling of specific tumors will provide information that will aid and refine the existing traditional morphologic classification of tumors. The World Health Organization (WHO) classification of breast tumors is probably the most widely used morphologic classification schema today, and will be used throughout this chapter.1
The grade of invasive carcinoma is a reflection of the degree of differentiation of the carcinoma. It is generally recognized that histologic grading provides prognostically important information and should be routinely performed for all types of invasive carcinomas of the breast.
The most widely used grading system for invasive breast carcinoma was developed in Nottingham, United Kingdom2 and is commonly referred to as the Nottingham Combined Histologic Grade.3 This grading system is a modification of the Bloom and Richardson grading system. There are 3 components of the histologic grade for invasive breast carcinoma:
Each component is given a numerical score of 1 to 3, with a score of 1 reflecting prominent gland formation, little nuclear atypia, and little mitotic activity, and 3 reflecting little or no gland formation, prominent nuclear atypia, and prominent mitotic activity. To obtain the overall grade, the score in each category is added to give a total out of 9 (6409118, 20-2, and 20-3):
Grading is performed after evaluation of all sections of carcinoma, and incorporates assessment of tubule formation throughout the carcinoma, degree of nuclear atypia from the most atypical areas, and mitotic activity from mitotic count of 10 high-power-fields from the most mitotically active area. Any combination of scores from these 3 features may exist. The high-power pictures in 6409118, 20-2, and 20-3 are representative of the invasive carcinomas; it should be remembered that accurate grading cannot be assigned from a single high-power view such as these.
The authors of this updated grading schema provide detailed criteria for assessment of the components of the grade, such as size of microscope field for counting of mitoses. Strict adherence to these criteria is necessary to attempt to reduce subjectivity and optimize reproducibility.3 Critics of grading stress its subjectivity, and many studies on reproducibility exist with wide-ranging results; however, pathology studies have shown that concordance in grading ranging up to 80% to >90% can be attained by strict adherence to uniform criteria.3,4 All references to grade within this chapter employ this grading scheme.
The proportion of carcinomas in the three grades (I, II, and III) is not equal, and the ratio of grade I:II:III is reported as 2:3:5.5
Certain factors are known to influence grading; these include tissue fixation, tumor heterogeneity, and size of sample.
Optimal tissue handling with prompt fixation is essential for preservation of histologic detail and to optimize grading. A decline in the number of recognizable mitotic figures in tissue has been correlated with delays in fixation, with a 30% reduction in mitotic figures with 2-hour delay in fixation and a 50% reduction with 6-hour delay in fixation.6 A reduction in mitotic count as a result of delayed fixation may result in underestimation of the grade of the invasive carcinoma. As formalin penetrates only a short distance into tissue (3.8 mm in 24 hours5), proper slicing of breast specimens to allow formalin penetration is essential. Submerging an intact specimen in formalin will not allow penetration of formalin into the tissue and will result in tissue changes secondary to delayed fixation.
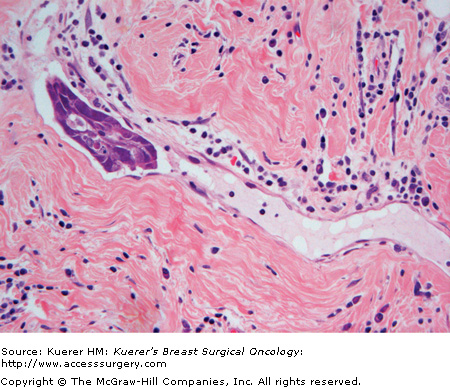
Peritumoral lymphovascular tumor emboli are identified in approximately 15% of invasive breast carcinomas after tissue examination by H&E staining (Fig. 20-4). While most patients with lymphovascular tumor emboli will also have axillary lymph node metastases, approximately 5% to 10% of patients who are axillary lymph node-negative by H&E have peritumoral lymphovascular tumor emboli. Multiple studies have shown that peritumoral lymphovascular tumor emboli are prognostically unfavorable.7-9 Care must be taken by the pathologist not to confuse spaces created by fixation retraction artifact around nests of tumor cells, with tumor in lymphovascular channels. Immunohistochemical stains for specific endothelial cell markers such as factor VIII, D2-40, or YLYVE-1 may assist in confirming the presence of lymphovascular channels, but we do not use these stains routinely, preferring to rely on routine H&E staining and strict morphologic criteria (ie, extratumoral location and convincing endothelial cell morphology) for this diagnosis.
Some routine immunohistochemical stains can be of great practical utility in the interpretation of breast carcinoma in identifying the presence of invasive/microinvasive carcinoma and assisting in the determination of the type of invasive carcinoma.10
There are situations in which the surgical pathologist has difficulty either making a diagnosis of invasive carcinoma or ruling it out, when using routine hematoxylin and eosin staining alone. Reasons for this include a complex morphology in which a benign process or in situ carcinoma may be mistaken for invasive carcinoma (for example, radial scar versus tubular carcinoma, or in situ carcinoma within sclerosing adenosis versus invasive carcinoma), or the presence of fibrosis and inflammation obscuring histologic detail. Particular difficulty may be experienced with certain pathologic entities such as papillary lesions, and with small biopsies such as cores in which the architectural context of the lesion is absent.
Immunohistochemical stains that are helpful in this context are markers of myoepithelium and epithelial cells. Myoepithelial cells are virtually always absent from around nests of invasive carcinoma, and present around benign and in situ lesions. Thus the absence of myoepithelium is very helpful in confirming suspected invasive carcinoma, and in contrast, its presence supports a lesion being either benign or in situ carcinoma. Commonly used myoepithelial markers include smooth muscle myosin heavy chain, calponin, smooth muscle actin, and P63. Epithelial markers such as LMWK and CK7 stain both benign and malignant epithelium and are very useful in demonstrating small numbers of invasive carcinoma cells obscured by inflammation, or in identifying small bland cells as being carcinoma, for example, with invasive lobular carcinoma.
E-cadherin is an epithelial specific cell–cell adhesion molecule11 encoded by a gene on chromosome 16q22.11 E-cadherin can be demonstrated in tissue by staining with anti–E-cadherin antibodies, and in the normal breast there is strong staining of the cell membranes of ducts and lobules.11 Ductal and lobular types of invasive carcinoma show different expression with anti–E-cadherin antibodies, with most invasive duct carcinomas showing strong membrane staining and most lobular carcinomas showing complete loss of membrane staining.11
Cytokeratins are intermediate filaments present in all epithelial and some nonepithelial cells. The type of cytokeratin may differ in carcinomas from different sites of origin, and in different morphologic patterns from the same tumor site. There is increasing interest in luminal- and basal-type cytokeratins in breast carcinoma. The following are luminal-type cytokeratins: CK7, CK8, CK18, and CK19. The following are basal-type cytokeratins: CK5/6, CK14, and CK17.12
Gene expression studies have revealed that breast carcinoma can be classified based on patterns of gene expression, and that this classification has clinical relevance.
Analysis of cDNA microarrays of samples of breast carcinoma by hierarchical clustering of patterns of gene expression has shown that breast carcinoma can be classified into groups based on profiles of gene expression.13-15 Two main subsets have been identified, an estrogen receptor (ER)-positive group characterized by higher expression of a panel of genes typically expressed by breast luminal cells (luminal cancer) and an ER-negative group that encompasses 3 subgroups: one group overexpressing ERBB2 (HER-2), one expressing genes characteristic of breast basal/myoepithelial cells (basal or basal-like cancer), and one with gene expression profile similar to normal breast tissue (normal breast-like).13-16 The ER-positive group is further subdivided into 2 or 3 subgroups—Luminal A, B, C in some classifications14,15; however, others have not defined 3 groups.16 This classification system has been found to have prognostic significance. Most studies show the best prognosis is in the luminal A group and the worst prognosis in the HER-2 and basal groups. The biologic significance of the normal breast-like group is not yet clear.16
Breast carcinomas do not all fit neatly into this classification: up to 50% of HER-2–positive tumors are hormone receptor–positive and up to 17% of hormone receptor-positive tumors are HER-2–positive.16
Use of the terminology “basal” and “basal-like” is not consistent; sometimes these terms are used interchangeably in the literature,17 while at other times the term “basal” is used to describe carcinoma identified by gene expression analysis studies, and “basal-like” or “with basal phenotype” to describe carcinomas with a particular immunostaining profile.
Expression of genes usually found in normal basal/myoepithelial cells of the breast remains the gold standard for identification of the basal type of breast carcinoma.17 As defined by gene microarray analysis, this accounts for up to 15% of breast cancer, often occurs in younger patients, is more frequent in African American women, and frequently shows lack of expression of hormone receptors and HER-2.17
These carcinomas usually express high-molecular-weight “basal” cytokeratins CK5/6, CK14, and CK17. Demonstration of staining with basal cytokeratins may be used as a surrogate for gene expression analysis to identify this type of carcinoma. There is no consensus as to what stain or stains should be used; however, the commonly used immunostains are cytokeratin 14 either alone or in combination with CK 5/6, ER, HER-2.16-18 Basal-like carcinoma is positive with cytokeratins 5/6 and 14 and usually negative with ER and HER-2.
Basal-like breast cancers are characterized by these morphologic features: high histologic grade with high mitotic index, pushing margin, central necrosis, metaplastic elements (squamous or spindle), and lymphocytic infiltrate.17,18 Several of these morphologic features are present in metaplastic and medullary types of invasive carcinoma, and it has been shown that >90% of metaplastic breast carcinomas and most medullary carcinomas show a basal-like phenotype.17
Basal-like breast cancers have been shown to have a more aggressive clinical behavior than non–basal-like breast cancers in some studies; however, other studies do not show a poorer outcome relative to either ER-negative non–basal-like cancer or grade-matched non–basal-like cancers.17 Some studies show basal-like breast cancers have a different pattern of metastatic spread, spreading to axillary nodes and bone less frequently, and showing hematogenous spread to brain and lung more frequently than the non-basal type.17,18
Breast carcinoma occurring in the BRCA1 germline mutation carrier, especially diagnosed before age 50, often has morphologic and immunostaining features similar to those described in basal-like breast cancer and is triple negative (ER, [progesterone receptor] PR, HER-2 negative).17
Triple-negative breast carcinomas account for 10% to 17% of breast carcinomas, occur more frequently in younger patients (< 50), are more prevalent in African American women, and often present as interval cancers17 in breast-screening programs. These carcinomas are mainly high-grade invasive duct carcinoma of no special type, metaplastic carcinoma, and medullary carcinomas.17
There is overlap between triple-negative and basal breast cancers. The groups are not the same17 as expression of at least one of ER, PR, and HER-2 is reported in 15% to 54% of basal-like breast cancers identified by microarray-based expression analysis. Furthermore, the triple-negative subgroup is not homogeneous; it includes the subset of breast cancer with “normal” gene expression pattern, which has a better prognosis than the basal-cell type.17
Invasive breast carcinoma of the “no special type” (NST) or “not otherwise specified” (NOS) variety makes up the largest single category of invasive breast carcinomas, accounting for between 47% and 75% of all invasive breast cancers, depending on the series.19,20 These tumors may present as a clinical lump, or be clinically inapparent and detected by a variety of imaging techniques including mammography, ultrasonography, or magnetic resonance imaging. The designation of a particular invasive mammary carcinoma as NST/NOS type is one of exclusion, and does not refer to a particular type of invasive ductal carcinoma but rather to an invasive ductal carcinoma that cannot be classified histologically as a particular special type of invasive ductal carcinoma based on current morphologic classification schemes (ie. the subtypes described later in the chapter). As such, NST tumors are a heterogeneous group of carcinomas of varying histologies and grades. The WHO definition of an NST tumor requires a nonspecial-type pattern to be present in greater than 50% of the tumor after a thorough examination of representative histologic sections. If an NST pattern is present in 10% to 49% of the tumor, the carcinoma is classified as mixed NST and special type. For an invasive carcinoma to classify as a special-type tumor, at least 90% of the tumor must show the appropriate special-type histomorphology. NST tumors appear to make up a lower proportion (approximately 40%) of small, screen-detected invasive carcinomas, and there may be a slightly increased frequency of NST tumors below the age of 35 years compared with older patients, who have an increased proportion of lobular and other special-type carcinomas.21
Generally, NST carcinomas form a solid, gray-white nodular mass that may be well circumscribed or have ill-defined, infiltrative borders on cut section. The descriptive terms “scirrhous” or “stellate” carcinoma refer to a carcinoma with extensive fibroelastosis that manifests grossly as firm yellow-white streaks on cut section.
Scirrhous carcinomas demonstrate a central solid mass that radiates irregularly into the surrounding fatty breast tissue. Tumor necrosis may be found in large, rapidly growing tumors that are outgrowing their blood supply, and this necrosis grossly presents as soft, white chalky areas. Gross cystic degeneration in NST tumors is rare.
The size of these tumors varies greatly, ranging from a few millimeters to over 10 centimeters; on average, the size of these tumors is approximately 2 cm in maximal dimension.
Microscopically, NST invasive ductal carcinoma is characterized by a growth of malignant epithelial cells organized into cohesive cords, nests, sheets, and tubules that infiltrate breast stroma in a disorganized and irregular fashion. The stroma within a particular tumor may be densely fibrotic or minimal in amount. The tumor cells of NST tumors show a varying degree of cytologic atypia, and variable amounts of inflammation may be present within/around the tumor mass. Immunostaining for myoepithelial cells (ie, with smooth-muscle myosin heavy-chain, calponin, or P63) or basement membrane (collagen IV) is often a useful adjunctive diagnostic technique to confirm the lack of a myoepithelial cell layer or basement membrane, a finding critical to the diagnosis of stromal invasion. Ductal carcinoma in situ (DCIS) is found in approximately 80% of cases either within or around the tumor mass, and is usually of intermediate or high grade.22 Approximately 70% to 80% of NST tumors are ER positive, 60% PR positive, and 15% to 30% are HER-2/neu positive.
The prognosis of a particular NST tumor is dependent on the traditional prognostic indicators such as size, tumor grade, lymphovascular invasion, and lymph node status. Overall, NST tumors show a 35% to 50% 10-year survival without adjuvant treatments. Following surgical excision with clear margins, treatment specifics (ie, postoperative radiation and chemotherapy) are determined by the traditional prognostic indicators noted earlier, as well as ER, PR, and HER-2/neu status of the tumor.
Invasive lobular carcinoma is the second most common type of invasive breast carcinoma after NST invasive duct carcinoma. The reported frequency is between 5% and 15%.23,24 Factors that contribute to this range include the wide range of morphologic features represented by invasive lobular carcinoma and differing criteria used for diagnosis, and the impact of mammographic screening and hormonal therapy. The incidence of invasive lobular carcinoma increased over the time period 1987 to 1999 while the incidence of invasive duct carcinoma remained stable. This increase occurred at a time of increased use of combined replacement hormonal therapy and it is postulated that the increased incidence of invasive lobular carcinoma is associated with this use.25 An increased proportion of lobular carcinoma has been reported in populations at higher risk of breast cancer.26
Several morphologic patterns of invasive lobular carcinoma are recognized, with the most common type termed the classic type. Other types are termed variants of invasive lobular carcinoma and include mixed, solid, alveolar, signet ring, histiocytoid, tubulolobular, and pleomorphic carcinoma. The types are differentiated based on histologic morphology, and some subtypes appear to be associated with differences in clinical outcome.
Patients with invasive lobular carcinoma tend to be slightly older than those with nonlobular invasive carcinoma (mean age reported as 57 years23 and 64 years27). On mammography, architectural distortion is more common and microcalcification less common with invasive lobular carcinoma compared to invasive duct carcinoma.1
Invasive lobular carcinoma tends to be larger at diagnosis than nonlobular invasive carcinoma,23 with 19% of invasive lobular carcinoma larger than 5 cm in one series.23 Increasing tumor size has been correlated with increasing grade.27 The gross appearance may be indistinguishable from invasive duct carcinoma with formation of a stellate mass, or may be more ill defined resulting in an area of vague induration that is difficult to delineate on gross examination. In the latter situation the invasive carcinoma may be much more extensive on microscopic examination than anticipated from gross examination.
A number of morphologic features distinguish invasive lobular from invasive duct carcinoma on microscopic examination; however, it is the combination of features that allows this distinction, as individually none of these features are specific for a lobular type in routine stains.28 Some invasive carcinomas (approximately 3-4%28) have features indeterminate between ductal and lobular type on routine stains. To accommodate this difficulty in classification, some pathologists have used terms such as “invasive carcinoma with both ductal and lobular features.” This is in addition to, and distinct from, mixed type of invasive carcinomas with features that can be identified as ductal in type in some areas and lobular in type in other areas—estimated to be 5% of invasive carcinoma.1 E-cadherin immunostaining is proving increasingly helpful in assigning a morphologic type since the majority of invasive lobular carcinomas do not show cytoplasmic membrane staining while invasive ductal carcinomas do.11,27
To qualify as an invasive lobular carcinoma, more than 90% of the carcinoma must show one of more patterns of lobular carcinoma, in keeping with the classification of other types of invasive mammary carcinoma. To qualify for a particular subtype there are not uniformly agreed-upon criteria, although an 80% threshold has been suggested (> 80% of a particular pattern required for a particular subtype designation, if < 80% considered mixed type).29
The most common subtypes of invasive lobular carcinoma are classical and mixed subtypes, representing 55% and 34% of a series of invasive lobular carcinoma, respectively.24
Some components of the grading scheme appear less applicable to invasive lobular carcinoma than nonlobular since the majority of invasive lobular carcinomas have a solid growth pattern and little mitotic activity.24 It is, however, recommended that invasive lobular carcinoma is graded using the same grading scheme as nonlobular carcinoma. The majority of invasive lobular carcinomas are grade II (76%2) with a small proportion being grade I or III (12% each24). Tubulolobular carcinoma and some classic invasive lobular carcinoma are grade I, pleomorphic invasive lobular carcinoma is grade II or III, and other classic invasive lobular carcinoma and variants are grade II. Invasive lobular carcinoma has been reported to be associated with an increased risk of multicentricity and bilaterality; however, some studies, both clinical and imaging, have not shown a significant difference in the incidence of multicentricity and bilaterality between invasive lobular carcinoma and invasive duct carcinoma.1,23 Invasive lobular carcinoma is more frequently ER positive than nonlobular carcinomas; the positivity rate ranges from 70% to 100%.1,27 PR positivity is similar to invasive duct carcinoma, in some reports positive in 60% to 70% of cases1; however, in some series invasive lobular carcinoma is more frequently PR positive than invasive duct carcinoma (positive in 85-90%27). HER-2 protein overexpression is less likely than with invasive duct carcinoma, with some reported series of invasive lobular carcinoma entirely negative (0% of 50 cases27); however, HER-2 protein overexpression have been reported in 81% of 38 cases of pleomorphic variant of invasive lobular carcinoma.30
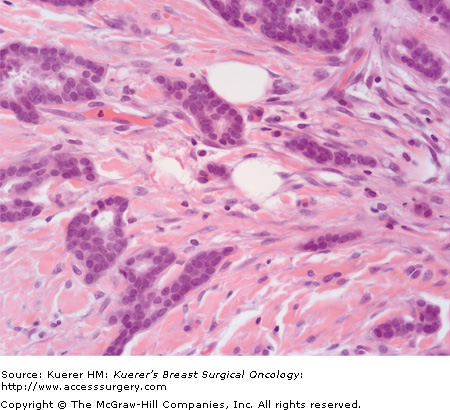
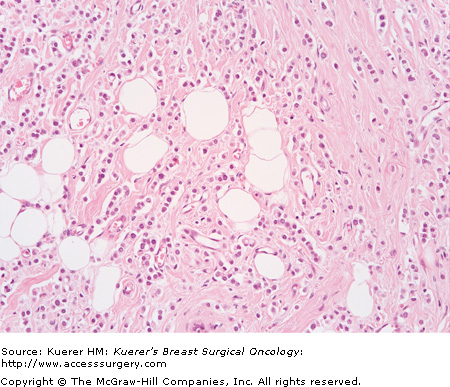
Classic invasive lobular carcinoma is the most common type of invasive lobular carcinoma. The tumor cells are small, uniform, and monotonous and infiltrate in a dispersed, noncohesive manner (Fig. 20-5). The cells often show a linear growth pattern (referred to as “single file”) and grow around structures in concentric lines of cells referred to as a “targetoid” growth pattern. The cells may percolate into fatty tissue between fat cells without associated stromal fibrosis. This growth pattern may result in the invasive carcinoma being more extensive than grossly estimated.
Small numbers of invasive tumor cells, particularly if admixed with lymphocytes or reactive changes, may easily be overlooked. These cells are keratin positive, which can be very helpful both to identify the existence of these cells and confirm that these are epithelial in nature.
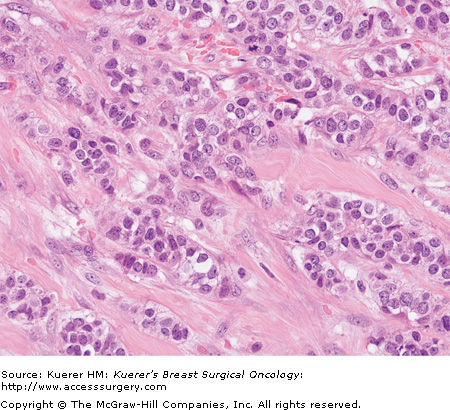
The tumor cells have the pattern of infiltration of classic lobular carcinoma but are larger, more pleomorphic, and have a tendency to aggregate, with features that may overlap with invasive ductal carcinoma (Fig. 20-6).30,31 Apocrine features may occur in invasive lobular carcinoma; this tends to be present in the pleomorphic variant,31 and these cells will be GCDFP-15 positive. Histiocytic features are also described in this variant.31
Figure 20-6

Invasive lobular carcinoma, pleomorphic type (H&E stain, 200×). In contrast to Figure 20–5, the tumor cells are more cohesive, forming small cell groups, and are larger with more nuclear variability and atypia.
There are no specific criteria for how large or atypical the tumor cells should be to qualify for this type, which will account for some variability in diagnosis. In general, the degree of mitotic activity identified is greater than with classic type. The use of E-cadherin stain allows identification of this as lobular type, which may otherwise have been interpreted as ductal type.
The tubulolobular variant of invasive lobular carcinoma is a rare variant with features of both tubular and lobular carcinoma. The pattern of infiltration is reminiscent of invasive lobular carcinoma with an infiltrative growth pattern and cells arranged in single file or in a targetoid growth pattern; however, the tumor cells also form small cohesive cell groups and microtubules.32 This has traditionally been classified as a variant of invasive lobular carcinoma; however, recently this type of invasive carcinoma has been found to show a ductal staining pattern with E-cadherin stain.32 Further studies will clarify if this should continue to be considered a variant of invasive lobular carcinoma or should be reclassified.1
The presence of some signet ring cells is frequent in forms of invasive lobular carcinoma, but extensive signet ring formation is much less frequent, representing 0.1% to 0.3% of invasive breast carcinoma.28
In the solid pattern the tumor cells are arranged in loose sheets, while in the alveolar pattern the tumor cells are in small, circumscribed aggregates of 20 or more cells and may simulate in situ lobular carcinoma.
The prognosis for invasive lobular carcinoma has been reported as worse, better, or the same as for invasive duct carcinoma.23 The prognostic value associated with tumor stage and lymph node status is applicable with invasive lobular carcinoma,23 but some have found less prognostic value with grade for invasive lobular carcinoma than with nonlobular types,23 possibly due to difficulty applying the grading scheme. With strict adherence to the criteria for grading, others have found histologic grade to be of independent prognostic value.24
The pleomorphic variant of invasive lobular carcinoma is associated with more aggressive clinical behavior than the classic type.31
Lymphoma is in the differential diagnosis of a breast mass composed of an infiltrate of noncohesive tumor cells. Staining with immunostains for keratin and lack of staining with lymphoid markers will allow this distinction.
Metastatic lobular carcinoma within lymph nodes is less easily recognizable than metastatic ductal carcinoma, as the cells may resemble histiocytes and be misinterpreted as a reactive change. Identification of metastatic lobular carcinoma at frozen section may be particularly difficult. Keratin immunostains may be very helpful in confirming metastatic lobular carcinoma in lymph node.
Tubular carcinoma is a special type of invasive duct carcinoma, which is well differentiated and has a favorable prognosis.
Overall, tubular carcinoma accounts for < 2% of invasive breast cancer.1 The proportion of invasive carcinomas of this type in different series is influenced both by the criteria used for histologic diagnosis and also the method of detection of the carcinoma. Tubular carcinoma is more common in cancers detected by mammographic screening than detected clinically, with the proportion of screen-detected cancers that are tubular carcinomas ranging from 9% to 19%.33 The majority of tubular carcinomas are detected mammographically34 as either a small spiculated mass or parenchymal deformity.35 Patients with tubular carcinoma are similar in age to patients with well-differentiated (grade I) invasive duct carcinoma.36,37
The majority of tubular carcinomas are 1 cm or less in maximum dimension.1,33,36 The mean dimension of tubular carcinoma has not changed over time; however, the mean dimension of invasive duct carcinomas has decreased over time,36 and currently the size of tubular carcinomas is similar to well-differentiated (grade I) invasive duct carcinoma.36
There are no distinguishing characteristics on gross examination.
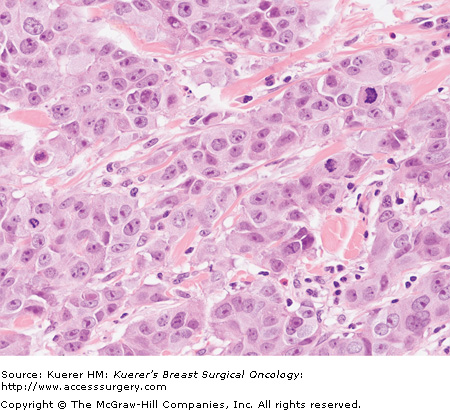
On microscopic examination, the invasive carcinoma is composed of glands (tubules) within stroma that is cellular and fibroblastic. The glands are open, irregular, and angulate in shape, and are lined by a single layer of epithelial cells, which may have protrusions into the lumen known as “apical snouts.” The tumor cells are small and uniform, without high-grade nuclear features, and mitoses are unusual (Fig. 20-7).
Figure 20-7
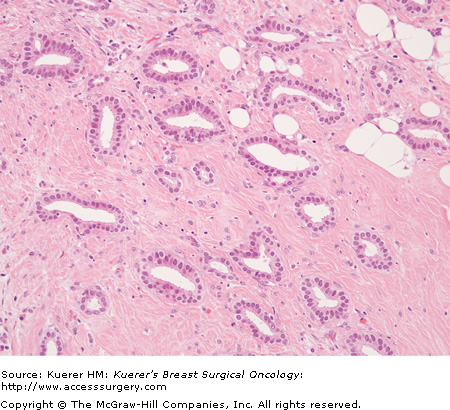
Tubular carcinoma (invasive carcinoma of tubular type) (H&E stain, 200×). The malignant cells are very bland in appearance and form well-defined glands. In some areas, the inner (luminal) surface of the glands have protrusions—”apical snouts.” The stroma shows some increased cellularity.
All tubular carcinomas are grade I invasive carcinomas, but the reverse is not so (not all grade I invasive carcinomas are tubular). Tubular carcinoma is differentiated from grade I invasive duct carcinoma mainly by the extent of tubule formation; there has not been uniformity in the proportion of carcinoma required to form tubular structures for a diagnosis of tubular carcinoma, which has ranged from at least 75% to 100%.1,37 The dominant current view is that at least 90% of the invasive carcinoma must form tubules to qualify for the designation of pure tubular carcinoma.1,3,37 Invasive carcinoma that is >50% tubular and the remainder cribriform is also classified as pure tubular carcinoma.33 Invasive carcinoma that is 50% to 90% tubular and the remainder of non-cribriform type is classified as mixed type of invasive carcinoma.1,33 Recent attempts have been made to refine the criteria for diagnosis by further evaluating tubule formation and also nuclear atypia and mitotic activity.36,38 Tubular carcinoma has been associated with a high risk of multicentricity (56%) and bilaterality (38%).39 The majority of tubular carcinomas are ER and PR positive, and negative for HER-2 protein over expression.1,39 The differential diagnosis of tubular carcinoma includes benign entities such as sclerosing adenosis and radial scar, and other forms of invasive carcinoma, particularly grade I invasive duct carcinoma of no special type, and also tubulolobular carcinoma. Differentiation from benign entities may be particularly challenging on core biopsies and is greatly assisted by the use of immunohistochemical stains for myoepithelium.
When uniform criteria for diagnosis are used, in comparison with well-differentiated (grade I) invasive duct carcinoma, patients with tubular carcinoma have a lower proportion of axillary node involvement at presentation (12.9% compared to 23.9%), a statistically significant lower rate of local recurrence, and a trend to lower rate of systemic relapse.37 The more restrictive the diagnostic criteria, the better the clinical outcome associated with a diagnosis of tubular carcinoma, with 100% 10-year disease-free survival reported in one series using the most restrictive diagnostic criteria.36
Mucinous carcinomas (also referred to as colloid carcinoma, gelatinous carcinoma, mucous carcinoma, or mucoid carcinoma) contain “large amounts of extracellular epithelial mucous sufficient to be visible grossly, and recognizable microscopically, surrounding and within tumor cells.”1 The tumor cells within this mucinous material must also demonstrate a low nuclear grade. As noted previously, by current WHO criteria, at least 90% of tumor must show this histomorphology to be classified as a pure mucinous carcinoma. Tumors that show mucinous differentiation in 50% to 90% of the tumor and/or high-grade nuclear cytology are classified as mixed NST/mucinous tumors, or NST tumors showing mucinous features, a diagnosis that does not connote an improved prognosis as does a pure mucinous carcinoma.
Pure mucinous carcinomas are relatively uncommon, accounting for approximately 2% of invasive ductal carcinomas when strict diagnostic criteria are applied.40 Most studies have noted a higher mean age for women with mucinous carcinoma as compared to patients with nonmucinous carcinoma. Mucinous carcinoma accounts for about 7% of carcinomas in women 75 years or older and only approximately 1% of women younger than 35 years of age.41 The majority of mucinous carcinomas present as a breast mass with mammographically detected microcalcifications occurring in a minority (ie, approximately 40%) of the tumors.42
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree