Objectives
- 1.
List the main endocrine glands of the body.
- 2.
List the chemical nature of the major hormones.
- 3.
Describe how the chemical nature influences hormone synthesis, storage, secretion, transport, clearance, mechanism of action, and appropriate route of exogenous hormone administration.
- 4.
Explain the significance of hormone binding to plasma proteins.
- 5.
Describe the major signal transduction pathways, and their mechanism for termination, for different classes of hormones and provide a specific example of each.
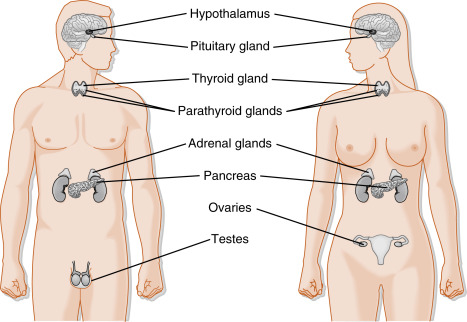
Endocrine glands secrete chemical messengers, called hormones ( Box 1.1 ), into the extracellular fluid in a highly regulated manner. Secreted hormones gain access to the circulation, often via fenestrated capillaries, and regulate target organs throughout the body. The endocrine system is composed of the pituitary gland , the thyroid gland , parathyroid glands , and adrenal glands ( Fig. 1.1 ). The endocrine system also includes the ovary and testis , which carry out a gametogenic function that is absolutely dependent on their endogenous endocrine function. In addition to dedicated endocrine glands, endocrine cells reside as a minor component (in terms of mass) in other organs, either as groups of cells (the islets of Langerhans in the pancreas) or as individual cells spread throughout several glands, including the gastrointestinal (GI) tract, kidney, heart, adipose tissue , and liver . In addition, there are several types of hypothalamic neuroendocrine neurons that produce hormones. The placenta serves as a transitory exchange organ, but also functions as an important endocrine structure of pregnancy.
Hormones Synthesized and Secreted by Dedicated Endocrine Glands
Pituitary Gland
Growth hormone (GH)
Prolactin
Adrenocorticotropic hormone (ACTH)
Thyroid-stimulating hormone (TSH)
Follicle-stimulating hormone (FSH)
Luteinizing hormone (LH)
Thyroid Gland
Tetraiodothyronine (T 4 ; thyroxine)
Triiodothyronine (T 3 )
Calcitonin
Parathyroid Glands
Parathyroid hormone (PTH)
Islets of Langerhans (Endocrine Pancreas)
Insulin
Glucagon
Somatostatin
Adrenal Gland
Epinephrine
Norepinephrine
Cortisol
Aldosterone
Dehydroepiandrosterone sulfate (DHEAS)
Hormones Synthesized by Gonads
Ovaries
Estradiol-17β
Progesterone
Inhibin
Testes
Testosterone
Antimüllerian hormone (AMH)
Inhibin
Hormones Synthesized in Organs with a Primary Function Other Than Endocrine
Brain (Hypothalamus)
Antidiuretic hormone (ADH; vasopressin)
Oxytocin
Corticotropin-releasing hormone (CRH)
Thyrotropin-releasing hormone
Gonadotropin-releasing hormone (GnRH)
Growth hormone–releasing hormone (GHRH)
Somatostatin
Dopamine
Brain (Pineal Gland)
Melatonin
Heart
Atrial natriuretic peptide (ANP)
Kidney
Erythropoietin
Adipose Tissue
Leptin
Adiponectin
Stomach
Gastrin
Somatostatin
Ghrelin
Intestines
Secretin
Cholecystokinin
Glucagon-like peptide-1 (GLP-1)
Glucagon-like peptide-2 (GLP-2)
Glucose-dependent insulinotropic peptide (GIP; gastrin inhibitory peptide)
Motilin
Liver
Insulin-like growth factor-I (IGF-I)
Hormones Produced to a Significant Degree by Peripheral Conversion
Lungs
Angiotensin II
Kidney
1α,25-dihydroxyvitamin D
Adipose, Mammary Glands, Other Organs
Estradiol-17β
Liver, Other Organs
Testosterone
Genital Skin, Prostate, Sebaceous Gland, Other Organs
5-Dihydrotestosterone (DHT)
Many Organs
T 3
The endocrine system also encompasses a range of specific enzymes, either cell-associated or circulating, that perform the function of peripheral conversion of hormonal precursors (see Box 1.1 ). For example, angiotensinogen from the liver is converted in the circulation to angiotensin I by the renal-derived enzyme renin, followed by conversion to the active hormone angiotensin II by the transmembrane ectoenzyme angiotensin I–converting enzyme (ACE) that is enriched in the endothelia of the lungs (see Chapter 7 ). Another example of peripheral conversion of a precursor to an active hormone involves the two sequential hydroxylations of vitamin D in hepatocytes and renal tubular cells.
Numerous extracellular messengers, including prostaglandins, growth factors, neurotransmitters, and cytokines, also regulate cellular function. However, these messengers act predominantly within the context of a microenvironment in an autocrine or paracrine manner, and thus are discussed only to a limited extent where needed.
To function, hormones must bind to specific receptors expressed by specific target cell types within target organs . Hormones are also referred to as ligands , in the context of ligand receptor binding, and as agonists , in that their binding to the receptor is transduced into a cellular response. Receptor antagonists typically bind to a receptor and lock it in an inactive state, unable to induce a cellular response. Drugs that bind to and alter the activity of steroid hormone receptors are referred to as selective receptor modulators. For example, Tamoxifen is a mixed estrogen receptor agonist/antagonist, and thus is referred to as a “ selective estrogen receptor modulator” or SERM . Loss or inactivation of a receptor leads to hormonal resistance . Constitutive activation of a receptor leads to unregulated, hormone-independent activation of cellular processes.
The widespread delivery of hormones in the blood makes the endocrine system ideal for the functional coordination of multiple organs and cell types in the following contexts:
- 1.
Allowing normal development and growth of the organism
- 2.
Maintaining internal homeostasis
- 3.
Regulating the onset of reproductive maturity at puberty and the function of the reproductive system in the adult
In the adult, endocrine organs produce and secrete their hormones in response to feedback control systems that are tuned to set-points , or set ranges, of the levels of circulating hormones. These set-points are genetically determined but may be altered by age, circadian rhythms (24-hour cycles or diurnal rhythms), seasonal cycles, the environment, stress, inflammation, and other influences.
Major forms of endocrine disease are caused by lack of hormone (e.g., hypothyroidism), excess of hormone (e.g., hyperparathyroidism) or dysfunction of receptor (hormonal resistance). It is important to appreciate that hormones often stimulate both the differentiated function and growth of target tissues and organs. This underlies the role of hormones in driving neoplastic transformation and cancer progression (i.e., the existence of hormonally responsive cancers). The pathogenesis of these and other forms of endocrine disease are discussed in the subsequent chapters.
The material in this chapter covers generalizations common to all hormones or to specific groups of hormones. The chemical nature of the hormones and their mechanisms of action are discussed. This presentation provides the generalized information necessary to categorize the hormones and to make predictions about the most likely characteristics of a given hormone. Some of the exceptions to these generalizations are discussed later.
Chemical Nature of Hormones
Hormones are classified biochemically as proteins/peptides, catecholamines, steroid hormones , and iodothyronines . The chemical nature of a hormone determines the following:
- 1.
How it is synthesized, stored, and released in a regulated manner
- 2.
How it is carried in the blood
- 3.
Its biologic half-life (t 1/2 ) and mode of clearance
- 4.
Its cellular mechanism of action
Proteins/Peptides
The protein and peptide hormones can be grouped into structurally related molecules that are encoded by gene families ( Box 1.2 ). Protein/peptide hormones gain their specificity from their primary amino acid sequence, which confers specific higher-order structures, and from posttranslational modifications, such as glycosylation.
- •
Synthesized as prehormones or preprohormones
- •
Stored in membrane-bound secretory vesicles (sometimes called secretory granules )
- •
Regulated at the level of secretion (regulated exocytosis) and synthesis
- •
Often circulate in blood unbound
- •
Usually administered by injection
- •
Hydrophilic and signal through transmembrane receptors
Protein/peptide hormones are synthesized on the polyribosome as larger preprohormones . The nascent peptides have at their N terminus a group of 15 to 30 amino acids called the signal peptide , which directs the growing polypeptide through the endoplasmic reticular membrane into the cisternae. The signal peptide is enzymatically removed, and the protein is then transported from the cisternae to the Golgi apparatus, where it is packaged into a membrane-bound secretory vesicle that buds off into the cytoplasm. Posttranslational modification occurs in the endoplasmic reticulum, Golgi apparatus, and secretory vesicle.
The original gene transcript is called either a prehormone or a preprohormone ( Fig. 1.2 ). Removing the signal peptide produces either a hormone or a prohormone. A prohormone is a polypeptide that requires further cleavage before the mature hormone is produced. Often this final cleavage occurs while the prohormone is within the Golgi apparatus or the secretory vesicle. Sometimes prohormones contain the sequence of multiple hormones. For example, the protein, proopiomelanocortin (POMC), contains the amino acid sequences of adrenocorticotropic hormone (ACTH) and α-melanocyte-stimulating hormone (αMSH). However, the pituitary corticotrope produces ACTH only, whereas keratinocytes and specific hypothalamic neurons produce αMSH, but not ACTH. The ability of cells to process the same prohormone into different peptides is due to cell type expression of prohormone convertases , resulting in cell-specific processing of the prohormone.
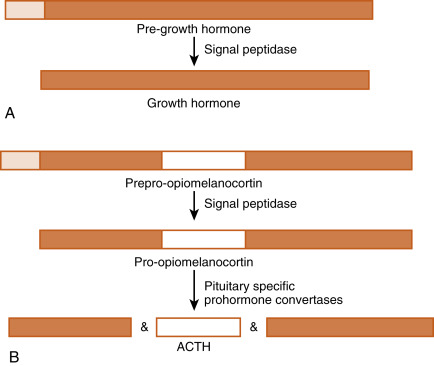
Protein/peptide hormones are stored in the gland as membrane-bound secretory vesicles and are released by exocytosis through the regulated secretory pathway . This means that hormones are not continually secreted, but rather that they are secreted in response to a stimulus, through a mechanism of stimulus-secretion coupling . Regulated exocytosis is induced by an elevation of intracellular Ca 2+ along with activation of other components (e.g., small G proteins), which interact with vesicular and cell membrane components. This ultimately leads to the fusion of the secretory vesicular membrane with the cell membrane and exocytosis of the vesicular contents.
Protein/peptide hormones are soluble in aqueous solvents and, with the notable exceptions of the insulin-like growth factors (IGFs) and growth hormone (GH), circulate in the blood predominantly in an unbound form; therefore they tend to have short biologic half-lives (t 1/2 ). Protein hormones are removed from the circulation by receptor-mediated endocytosis and lysosomal turnover of hormone receptor complexes (see later). Many protein hormones are small enough to appear in the urine in a physiologically active form. For example, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are present in urine. Pregnancy tests using human urine are based on the presence of the placental LH-like hormone, human chorionic gonadotropin (hCG).
Proteins/peptides are readily digested if administered orally. Hence, they must be administered by injection or, in the case of small peptides, through a mucous membrane (sublingually or intranasally). Because proteins/peptides do not cross cell membranes readily, they signal through transmembrane receptors .
Catecholamines
Catecholamines are synthesized by the adrenal medulla and neurons and include norepinephrine, epinephrine, and dopamine ( Fig. 1.3 ; Box 1.3 ). The primary hormonal product of the adrenal medulla is epinephrine , and to a lesser extent, norepinephrine. Epinephrine is produced by enzymatic modifications of the amino acid tyrosine . Epinephrine and other catecholamines are ultimately stored in secretory vesicles that are part of the regulated secretory pathway. Epinephrine is hydrophilic and circulates either unbound or loosely bound to albumin. Epinephrine and norepinephrine are similar to protein/peptide hormones in that they signal through membrane receptors, called adrenergic receptors . Catecholamines have short biologic half-lives (a few minutes) and are inactivated by intracellular enzymes. Inactivated forms diffuse out of cells and are excreted in the urine.
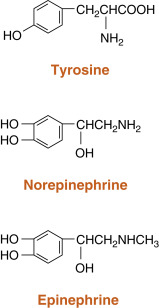
- •
Derived from enzymatic modification of tyrosine
- •
Stored in membrane-bound secretory vesicles
- •
Regulated at the level of secretion (regulated exocytosis) and through the regulation of the enzymatic pathway required for their synthesis
- •
Transported in blood free or only loosely associated with proteins
- •
Often administered as an aerosol puff for opening bronchioles, and several specific analogs (agonists and antagonists) can be taken orally
- •
Hydrophilic and signal through transmembrane G-protein-coupled receptors called adrenergic receptors
Steroid Hormones
Steroid hormones are made by the adrenal cortex, ovaries, testes , and placenta ( Box 1.4 ). Steroid hormones from these glands fall into five categories: progestins, mineralocorticoids, glucocorticoids, androgens , and estrogens ( Table 1.1 ). Progestins and the corticoids are 21-carbon steroids, whereas androgens are 19-carbon steroids and estrogens are 18-carbon steroids. Steroid hormones also include the active metabolite of vitamin D , which is a secosteroid (see Chapter 4 ).
- •
Derived from enzymatic modification of cholesterol
- •
Cannot be stored in secretory vesicles because of lipophilic nature
- •
Regulated at the level of the enzymatic pathway required for their synthesis
- •
Transported in the blood bound to transport proteins (binding globulins)
- •
Signal through intracellular receptors (nuclear hormone receptor family)
- •
Can be administered orally
| Family | No. of Carbons | Specific Hormone | Primary Site of Synthesis | Primary Receptor |
|---|---|---|---|---|
| Progestin | 21 | Progesterone | Ovary placenta | Progesterone receptor (PR) |
| Glucocorticoid | 21 | Cortisol, Corticosterone | Adrenal cortex | Glucocorticoid receptor (GR) |
| Mineralocorticoid | 21 | Aldosterone, 11-Deoxycorticosterone | Adrenal cortex | Mineralocorticoid receptor (MR) |
| Androgen | 19 | Testosterone, Dihydrotestosterone | Testis | Androgen receptor (AR) |
| Estrogen | 18 | Estradiol-17β, Estriol | Ovary placenta | Estrogen receptor (ER) |
Steroid hormones are synthesized by a series of enzymatic modifications of cholesterol ( Fig. 1.4 ). The enzymatic modifications of cholesterol are of three general types: hydroxylations, dehydrogenations/hydrogenations, and breakage of carbon-carbon bonds. The purpose of these modifications is to produce a cholesterol derivative that is sufficiently unique to be recognized by a specific receptor. Thus progestins bind to the progesterone receptor (PR) , mineralocorticoids bind to the mineralocorticoid receptor (MR) , glucocorticoids bind to the glucocorticoid receptor (GR) , androgens bind to the androgen receptor (AR) , estrogens bind to the estrogen receptor (ER) , and the active vitamin D metabolite binds to the vitamin D receptor (VDR) .
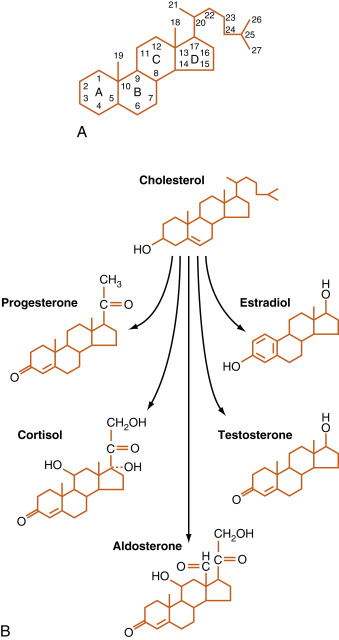
The complexity of steroid hormone action is increased by the expression of multiple forms of each receptor. Additionally, there is some degree of nonspecificity between steroid hormones and the receptors they bind to. For example, glucocorticoids bind to the MR with high affinity, and progestins, glucocorticoids, and androgens can all interact with the PR, GR, and AR to some degree. An appreciation of this “cross-talk” is important to the physician who is prescribing synthetic steroids. For example, medroxyprogesterone acetate (a synthetic progesterone given for hormone replacement therapy in postmenopausal women) binds well to the AR as well as the PR. As discussed subsequently, steroid hormones are lipophilic and pass through cell membranes easily. Accordingly, classic steroid hormone receptors are localized intracellularly and act by regulating gene expression. More recently, membrane and juxtamembrane receptors have been discovered that mediate rapid, nongenomic actions of steroid hormones.
Steroidogenic cell types are defined as cells that can convert cholesterol to pregnenolone , which is the first reaction common to all steroidogenic pathways. Steroidogenic cells have some capacity for cholesterol synthesis but often obtain cholesterol from circulating cholesterol-rich lipoproteins (low-density lipoproteins and high-density lipoproteins; see Chapter 3 ). Pregnenolone is then further modified by six or fewer enzymatic reactions. Because of their hydrophobic nature, steroid hormones and precursors can leave the steroidogenic cell easily and so are not stored. Thus steroidogenesis is regulated at the level of uptake, storage, and mobilization of cholesterol and at the level of steroidogenic enzyme gene expression and activity. Steroids are not regulated at the level of secretion of the preformed hormone. A clinical implication of this mode of secretion is that high levels of steroid hormone precursors are easily released into the blood when a downstream steroidogenic enzyme within a given pathway is inactive or absent ( Fig. 1.5 ). In comparing the ultrastructure of a protein hormone–producing cell to that of a steroidogenic cell, protein hormone–producing cells store the product in secretory granules and have extensive rough endoplasmic reticula. In contrast, steroidogenic cells store the precursor (cholesterol esters) in the form of lipid droplets, but do not store the product. Steroidogenic enzymes are localized to smooth endoplasmic reticulum membrane and within mitochondria, and these two organelles are numerous in steroidogenic cells.
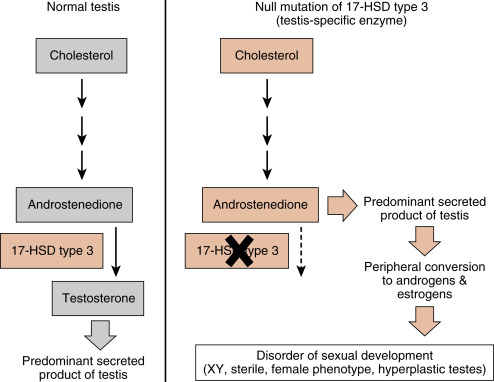
An important feature of steroidogenesis is that steroid hormones often undergo further modifications (apart from those involved in deactivation and excretion) after their release from the original steroidogenic cell. This is referred to as peripheral conversion . For example, estrogen synthesis by the ovary and placenta requires at least two cell types to complete the pathway of cholesterol to estrogen (see Chapter 10 , Chapter 11 ). This means that one cell secretes a precursor, and a second cell converts the precursor to estrogen. There is also considerable peripheral conversion of active steroid hormones. For example, the testis secretes sparingly little estrogen. However, adipose, muscle, and other tissues express the enzyme for converting testosterone (a potent androgen) to estradiol-17β. Peripheral conversion of steroids plays an important role in several endocrine disorders (e.g., see Fig. 1.5 ).
Steroid hormones are hydrophobic, and a significant fraction circulates in the blood bound to transport proteins (see later). These include albumin, but also the specific transport proteins, sex hormone–binding globulin (SHBG) and corticosteroid-binding globulin (CBG) (see later). Excretion of hormones typically involves inactivating modifications followed by glucuronide or sulfate conjugation in the liver. These modifications increase the water solubility of the steroid and decrease its affinity for transport proteins, allowing the inactivated steroid hormone to be excreted by the kidney. Steroid compounds are absorbed fairly readily in the GI tract and therefore often may be administered orally.
Thyroid Hormones
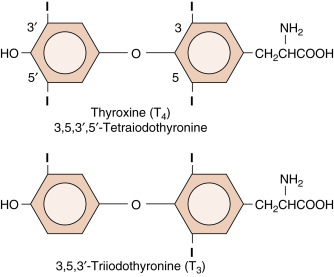
Thyroid hormones are classified as iodothyronines ( Fig. 1.6 ) that are made by the coupling of iodinated tyrosine residues through an ether linkage ( Box 1.5 ; also see Chapter 6 ). Their specificity is determined by the thyronine structure, but also by exactly where the thyronine is iodinated. Normally, the predominant iodothyronine released by the thyroid is T 4 (3,5,3′,5′-tetraiodothyronine , also called thyroxine) , which acts as a circulating precursor of the active form, T 3 (3,5,3′-triiodothyronine) . Thus peripheral conversion through specific 5 ′- deiodination plays an important role in thyroid function (see Chapter 6 ). Thyroid hormones cross cell membranes by both diffusion and transport systems. They are stored extracellularly in the thyroid as an integral part of the glycoprotein molecule thyroglobulin (see Chapter 6 ). Thyroid hormones are sparingly soluble in blood and are transported in blood bound to thyroid hormone–binding globulin (TBG) . T 4 and T 3 have long half-lives of 7 days and 24 hours, respectively. Thyroid hormones are similar to steroid hormones in that the thyroid hormone receptor (TR) is intracellular and acts as a transcription factor. In fact, the TR belongs to the same gene family that includes steroid hormone receptors and VDRs. Thyroid hormones can be administered orally and sufficient hormone is absorbed intact to make this an effective mode of therapy.
- •
Derived from the iodination of tyrosines, which are coupled to form iodothyronines
- •
Lipophilic, but stored in thyroid follicle cells by covalent attachment to thyroglobulin
- •
Regulated at the level of synthesis, iodination, and secretion
- •
Transported in blood tightly bound to proteins
- •
Signal through intracellular receptors (nuclear hormone receptor family)
- •
Can be administered orally
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree