Fig. 3.1
Formation of tissues and germ layer derivatives
3.3.1 Ectoderm
Ectoderm produces the epidermis of the skin and its appendages (i.e., hair follicles and sebaceous glands), the cornea and lens of the eye and the epithelial linings of the mouth, nasal cavity, salivary glands, and anal canal. Portions of the skull, teeth, and the adrenal medulla are also derived from ectoderm together with the nervous system (including the hypothalamus, pituitary, and pineal gland) [1, 2].
3.3.2 Mesoderm
3.3.3 Endoderm
This inner layer produces most of the epithelium of the gastrointestinal tract and its organs (i.e., liver, gallbladder, and pancreas), the lining of the respiratory system (i.e., trachea, bronchi, and alveoli) the bladder and portions of the urethra. The thymus gland, thyroid gland, and parathyroid glands also develop from the endoderm layer [1, 2].
3.4 Cellular Adaptation and Death
Cells constantly adapt within a narrow range of physiological parameters so that internal conditions remain relatively constant. This is referred to as cellular “homeostasis” [3]. Cells are capable of making changes in response to unfavorable environmental changes (i.e., injury) in an attempt to maintain this internal stability, which may be physiological (or normal) or pathological (or abnormal) changes [4]. The most common types of cellular adaptation include cell atrophy, hypertrophy, hyperplasia, and metaplasia and are illustrated in Fig. 3.2.
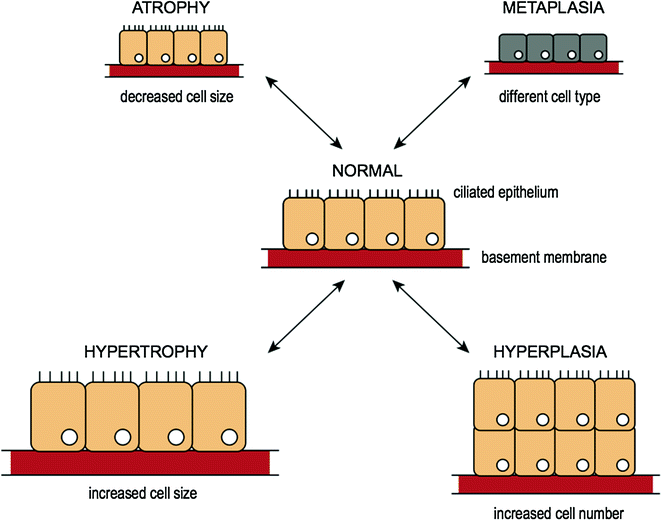

Fig. 3.2
Reversible cell injury and cellular adaptation
3.4.1 Atrophy
Atrophy is the reduction in size of cells in response to cell injury [5]. It results from decreased protein synthesis and increased protein degradation within the cells. Atrophy of various cells and organs is normal at certain points in the human life cycle. An example of atrophy as part of normal development includes the involution of the thymus gland in early childhood. Examples of pathologic causes of atrophy include atrophying muscle diseases (e.g., muscular dystrophy [6] or myotonic dystrophy [7]) characterized by progressive muscle weakness over time resulting in functional disability [6, 7].
3.4.2 Hypertrophy and Hyperplasia
Hypertrophy refers to an increase in volume of an organ or tissue due to the enlargement of cell size. There is no increase in cell number. Hypertrophy should be distinguished from hyperplasia where cells remain approximately the same size but increase in number, although both can occur simultaneously. In hypertrophy, cells are enlarged by an increased amount of structural proteins and organelles. This can be both physiologic or pathologic due to increased functional requirements. A physiological example of hypertrophy is increased muscle size in response to normal exercise [8, 9] (e.g., lifting weights), whereas a pathologic example would include cardiac enlargement as a result of hypertension [10, 11]. An example of a normal hyperplastic response on the other hand, is the normal proliferation of glandular epithelium in the breast as a response to pregnancy [12]. A pathologic example of hyperplasia would include endometrial hyperplasia as a result of unopposed estrogen [13], a risk factor for endometrial carcinoma [14].
3.4.3 Metaplasia
The definition of metaplasia is the change of a cell from one cell type to another cell type. This occurs when the original cell type is unable to withstand new environmental stress and changes into another phenotype more suited to the new environment. Metaplasia is usually reversible once the cause of environmental stress is removed. An example of metaplasia is intestinal metaplasia, whereby the change in cell type resembles that found in the intestine (i.e., intestinalized columnar epithelium with goblet cells). When intestinal metaplasia occurs in the esophagus [15] (normally lined by squamous mucosa), it is referred to as “Barrett’s esophagus” [16]. This diagnosis is clinically important as these patients are at risk of developing esophageal adenocarcinoma [17], and therefore it is considered to be a premalignant condition.
3.4.4 Cell Death: Necrosis and Apoptosis
If the injury to the cell is too severe (i.e., irreversible injury), the affected cells are no longer able to adapt to stress and subsequently die [18]. There are two main types of cell death, necrosis [19], and apoptosis [20]. These differ in their roles in disease and in their mechanisms [18] and are compared in Fig. 3.3. Cellular necrosis is caused by factors outside the cell (e.g., infection, toxins, or trauma) that result in the unregulated breakdown of the cell’s internal components. When the damage to a cell’s plasma membrane is severe, enzymes leak from lysosomes into the cytoplasm and cause autolysis [21]. The leakage of cellular contents through the damaged cell membrane prompts a host reaction (i.e., an inflammatory response). In contrast, apoptosis (or programmed cell death) is a targeted cause of cellular demise [22] where activated enzymes degrade the internal contents of the cell. Although the plasma membrane remains intact, it becomes altered so that it fragments into apoptotic bodies which ultimately become phagocytosed by host immune cells [23]. In this form of cellular death an inflammatory response is not elicited [20].
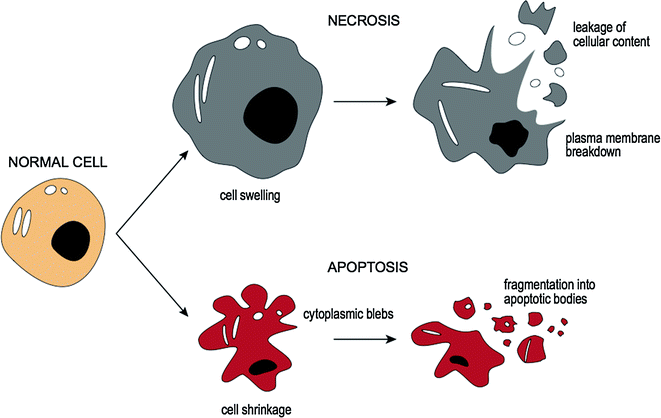

Fig. 3.3
Cellular death: necrosis and apoptosis
3.5 Dysplasia and Carcinoma In Situ
Dysplasia is the abnormal growth or development of cells where the cells are structurally changed in shape, size, and appearance from the original cell type. The tissue becomes disordered in appearance under the microscope, often with an increase in the number of immature cells. Dysplasia is often a precursor to tumor formation (or neoplasia) [24–26] and the chances of development into a carcinoma rise with worsening degrees of dysplasia. Low grade cervical dysplasia usually does not usually evolve into cervical carcinoma for example, whereas high grade dysplasia usually does [27, 28]. High grade, or full thickness dysplasia is often referred to as “carcinoma in situ” and refers to neoplastic cells within the normal boundaries of the tissue of origin (i.e., neoplastic cells do not migrate beyond the basement membrane). This is often abbreviated as CIS and some refer it as “pre-cancer” or “non-invasive cancer” [29]. In contrast, invasive carcinoma refers to when neoplastic cells have moved beyond the basement membrane layer [30] and therefore have the potential to spread to other areas within the body. The progression from normal epithelium to invasive carcinoma is illustrated in Fig. 3.4.
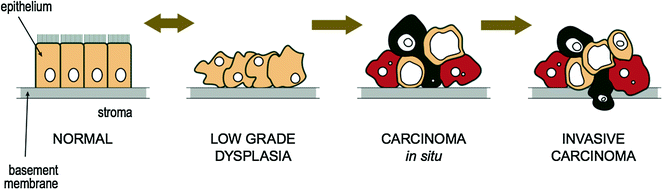

Fig. 3.4
Progression from normal epithelium to invasive carcinoma
3.6 Neoplasia
Neoplasia is the process of tumor formation resulting in a neoplasm (or tumor). Tumors are classified as either benign or malignant based on their pathology and known clinical behaviors [5]. It is very important for a histopathologist to be able to distinguish between a benign and a malignant tumor, as the treatment required is usually very different. Certain long-established histological features indicate innocence while other features indicate malignancy, therefore the distinction between the two tumor classifications is made with remarkable accuracy. Histopathologic features assessed include evaluation of architectural (i.e., the arrangement of cells) and cytological characteristics (i.e., the nuclear and cytoplasmic features) of the tissue under examination. The normal architecture of an organ involves standard relationships between specific groups of cells. In neoplastic growth, the relationships between cells are significantly distorted. The architecture of neoplastic cells in comparison to normal cells is not orderly and many layers of cells are arranged in irregular patterns. Various malignancies show particular altered architectural growth patterns also, for example, a “papillary” (or finger-like) growth pattern in thyroid carcinoma [31, 32] in comparison to normal thyroid follicles lined by a single layer of cuboidal epithelium [33]. Other histological patterns of growth are listed in Table 3.1.
Table 3.1
Histological patterns of growth
Histological pattern | Description |
|---|---|
Papillary | “Finger-like” projections lined by neoplastic cells with central fibrovascular cores |
Cribriform | Clusters of neoplastic cells with sharply punched out round spaces, described as “swiss cheese-like” |
Solid | Sheets of multiple neoplastic cells together with little intervening stroma |
Nested | Small solid groups or “nests” of neoplastic cells which cluster together |
Infiltrative | Neoplastic cells growing in single-file and intersecting into surrounding structures |
Rosettes | Neoplastic cells growing circumferentially around a lumen forming a “halo-like” or “spoke-wheel” shape |
Whorls | Neoplastic cells sweeping and swirling in different directions |
Fascicular | Bundles of neoplastic cells intersecting at right angles to each other |
Neoplastic cells are also distinguished from normal cells by a loss of “cellular differentiation” [4, 5]. Differentiation refers to how different the tumor cells are from the cells from which they originated. If neoplastic cells are almost like normal cells they are said to be “well differentiated.” In contrast, if they are very unlike normal cells they are referred to as “poorly differentiated.” In pathology, tumor grade is a measure of cellular differentiation [34] where low grade tumors are well differentiated, high grade tumors are poorly differentiated and intermediate grade tumors are intermediate between these two. Each type of cancer is graded using a different system, e.g., the Gleason grading system [35, 36] for prostate carcinoma or the Nottingham grading system [37, 38] for breast cancer.
The cytological changes seen in tumors include increased variations in cellular and nuclear size and shape (or pleomorphism) compared to corresponding normal cells, large hyperchromatic (i.e., stain more deeply basophilic) nuclei containing coarse, irregular chromatin, increased nuclear to cytoplasmic ratios, large nucleoli, and a high rate of cell division according to the number of cells with the nucleus showing the characteristic pattern of separating chromosomes (or mitotic figures [39]).
3.6.1 Benign Tumors
Benign lesions refer to non-cancerous, localized tumors, which do not have the capacity to spread to other sites and are generally amenable to surgical removal. These tumors are named by the cell type from which they originate, followed by the suffix “-oma.” An example is a “lipoma” [40], a benign tumor of fat cells (or lipocytes) [41]. Other examples of benign tumors are listed in Table 3.2. Benign tumors tend to be histologically and cytologically similar to their tissues of origin (i.e., they are well differentiated). However, the gross structure of a benign tumor may stray from the normal and assume papillary or polypoid configurations, for example, in squamous papillomas of the skin [42] in comparison to the flat epidermal layer of normal skin [33]. Once excised, benign tumors do not tend to recur or spread to distant sites.
Table 3.2
Examples of benign and malignant neoplasms and relationship to cell of origin
Neoplasm | |||
|---|---|---|---|
Cell origin | Cell type | Benign | Malignant |
Ectoderm | Skin cells | Nevus | Malignant melanoma |
Nerve cells | Neurofibroma | Neurofibrosarcoma | |
Mesoderm | Fat cells | Lipoma | Liposarcoma |
Cartilage cells | Chondroma | Chondrosarcoma | |
Bone | Osteoma | Osteogenic sarcoma | |
Blood vessels | Hemangioma | Angiosarcoma | |
Lymph vessels | Lymphangioma | Lymphangiosarcoma | |
Smooth muscle | Leiomyoma | Leiomyosarcoma | |
Striated muscle | Rhabdomyoma | Rhabdomyosarcoma | |
Hematopoietic cells | NA | Lymphoma/leukemia | |
Endoderm | Colonic cells | Adenoma | Adenocarcinoma |
Liver cells | Liver cell adenoma | Hepatocellular carcinoma | |
3.6.2 Malignant Tumors
Malignant lesions are cancers which show aggressive behavior including invasion into and destruction of adjacent tissues. These tumors can also recur if incompletely excised and also have the capacity to spread to other sites. There are two general pathologic categories including “carcinomas” derived from epithelial cells (i.e., from endodermal or ectodermal layers) or “sarcomas” of mesenchymal origin (i.e., from the mesodermal layer). Lymphomas and leukemias are a separate category of malignancies that arise from hematopoietic cells. Malignant lesions are further defined by their tissue of origin (i.e., prostatic [43]). Some examples of malignant tumors are listed in Table 3.2. Malignant neoplasms exhibit malignant cytologic features, disorganized growth patterns, abundant mitotic figures, and arrangements around blood vessels. Malignant tumors often outgrow their blood supply and show ischemic necrosis. The rate of growth of malignant lesions usually correlates inversely with their level of differentiation.
3.7 Important Biological Characteristics of Solid Tumors
Tumors are believed to be acquired through a multistep process involving genetic alterations which result in the loss of function of genes which normally inhibit cell growth (i.e., tumor suppressor genes) [44], increased activation of genes that stimulate proliferation (i.e., oncogenes) [45] or alteration of the function of genes involved in DNA repair [46]. Genes can be mutated in several different ways including altered arrangement of chromosomes (or rearrangement) [47] resulting in translocations [48], insertions [49], deletions or duplications [47], which may lead to subsequent gene activation or inactivation. Mutations are seen in many important regulatory pathways including genes that activate and deactivate carcinogens and those that govern the cell cycle, cell senescence (or aging), apoptosis, angiogenesis (or new blood vessel formation), cell signaling, and cellular differentiation to name a few.
3.7.1 Proliferative Activity
The normal human cell cycle involves a complex system of signaling pathways that a cell undergoes in order to copy itself precisely [50]. As illustrated in Fig. 3.5, the normal human cell cycle is divided into four phases: the first growth phase (G1), the DNA synthesis phase (S), the second growth phase (G2), and mitosis (i.e., the cellular division phase) (M). The cycle is checked at three main checkpoints: the G1/S checkpoint, the G2/M checkpoint, and the M checkpoint [50]. If a cell reaches a checkpoint and damage is detected, the cell cycle ceases for a time. During this time, the cell has the opportunity to repair the DNA damage using various DNA repair genes and resume cycling [51]. If this repair is not successful, the cell is then triggered into apoptosis. In contrast, in the tumor cell cycle [50, 52], neoplastic cells may undergo multiple consecutive cycles of mitosis [53] or they may leave the cell cycle, remain dormant for a period and reenter the cycle with the appropriate stimulus.
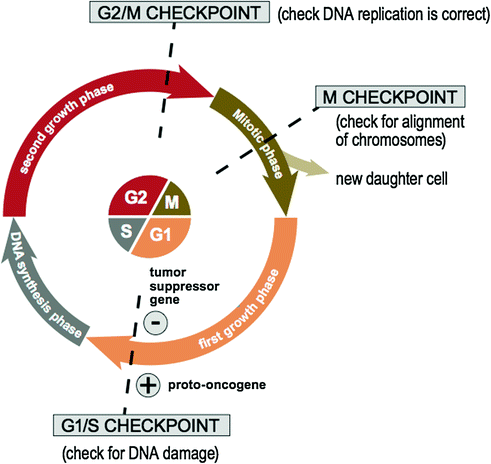

Fig. 3.5
The normal human cell cycle
Proto-oncogenes and tumor suppressor genes are important components of the cell cycle involved in checkpoint control. Firstly, proto-oncogenes (e.g., RAS [54], MYC [55], CDK [56], etc.) encode components of cellular proliferation within the cell cycle including growth factors, receptors, signaling enzymes, and transcription factors. Oncogenes arise from the mutation or increased expression of proto-oncogenes [57] and disrupt the cell’s normal signaling pathway allowing for uncontrolled cell proliferation. In contrast, tumor suppressor genes (e.g., TP53 [58], PTEN [59], etc.) are a family of genes that instruct cells to produce proteins that inhibit cell growth within the cell cycle. The loss of these proteins allows a cell to grow and divide in an uncontrolled manner [60]. Finally, DNA repair genes are also essential components of the cell cycle and code for proteins whose normal function is to correct errors that arise during DNA duplication. There are multiple DNA repair genes and various pathways including base excision repair (BER) [61], nucleotide excision repair (NER) [62], homologous recombination (HR) repair [63], and mismatch repair (MMR) [64]. Mutations in DNA repair genes involved in these pathways can lead to a failure in DNA repair, which in turn allows subsequent mutations to accumulate. An example is a hereditary mutation of MMR genes (i.e., Lynch syndrome) [65] leading to a high lifetime risk of colonic carcinoma [66] and other cancers.
3.7.2 Tumor Stroma
In general, neoplasms are composed of two main components, the tumor parenchyma (i.e., the main tumor mass composed of proliferating tumor cells) and the tumor stroma (i.e., the microenvironment around the tumor). In addition to all the molecular changes that occur within a cancer cell, the tumor stroma is thought to also undergo alterations [67] and contribute both to tumorigenesis and resistance to chemotherapeutic agents. Stroma consists of a supportive framework, which includes the basement membrane, fibroblasts, inflammatory cells, vascular cells, and the extracellular matrix (ECM) [68]. Fibroblasts represent the principal cellular stromal component [69]. Normal fibroblasts are usually inactive within the ECM. Once they are recruited and activated around the tumor cells, they are referred to as cancer-associated fibroblasts (CAFs) [70] or tumor-associated fibroblasts and have different activity (e.g., higher proliferative activity) in comparison to normal fibroblasts. Some authors report that tumor cells are thought to cause the proliferation of CAFs and secretion of collagen (i.e., the reactive stroma hypothesis). Others believe that tumor cells dedifferentiate into CAFs and then secrete collagens (i.e., the tumor-induced change hypothesis). Under the microscope, this distinctive fibrous response (or desmoplastic reaction) can vary from a very dense hyalinised stroma with a minimal cellular infiltrate to a predominantly cellular stroma with little collagenous tissue. Certain malignancies are known to have a more pronounced desmoplastic reaction (e.g., breast cancer [71] or pancreatic cancer [72]) than others (e.g., colon cancer).
Angiogenesis is another important feature of cancer progression [73]. It begins when neoplastic cells secrete molecules (e.g., VEGF) [74] into the surrounding stroma activating vascular endothelial cells, which in turn produce enzymes (matrix metalloproteinases) to breakdown the ECM. This permits migration and division into networks of blood vessels. Multiple proteins have been identified as angiogenic activators [73] including VEGF, FGF, TGF beta, EGF, PDGF, etc. For many years, researchers have concentrated on the malignant tumor cells as the main target of cancer therapy with the majority of chemotherapeutics either selectively killing tumor cells (cytotoxic) or restricting tumor growth. In recent years, however, the tumor stroma is now being pursued as an anti-cancer target itself [75]. Consequently, stromal molecules are being targeted by inhibitors, e.g., CAF inhibitors [76] (e.g., PDGF receptor inhibitors [77] like Dasatinib [78]) or angiogenesis inhibitors [79] (e.g., VEGF receptor inhibitors [80, 81] like Bevacizumab [82]).
3.7.3 Tumor Tissue Heterogeneity
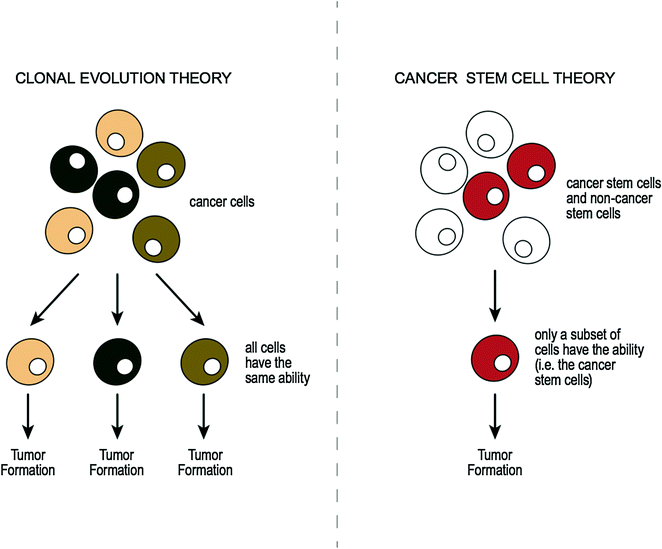
Intratumoral heterogeneity (i.e., coexistence of distinct clones within a tumor) has been the subject of much debate. Authors have shown that tumor subclones may differ from the original clone in various characteristics [83], for example in invasiveness [84], in metastatic potential [85], and in response to chemotherapy [86]. Also it is thought that primary and metastatic lesions can also be genetically distinct [87, 88], which again may affect patient therapeutic options. Two concepts have been proposed as causes for tumor phenotypic heterogeneity, “the clonal evolution theory” (or “the conventional cancer theory”) [83] and “the cancer stem cell theory” [89]. Simplistic illustrations comparing each of these theories are outlined in Fig. 3.6. The conventional cancer theory proposes that any cancer cell within a tumor can accumulate mutations and initiate tumor growth. In this multistep theory, a series of clonal expansions occurs, each of which is triggered by the acquisition of further mutations allowing mutant clone to expand [83]. In contrast, the stem cell theory suggests that among all cancerous cells within a tumor, only a subset of cells act as stem cells [89]. Normal stem cells are cells that can reproduce themselves and give rise to other kinds of cells. Cancer stem cells (CSC) are therefore cancerous cells that possess the same characteristics as are associated with normal stem cells. Research has shown that cancerous cells within a tumor may, therefore, not all be the same, in that some may be CSCs while others may be non-CSCs [90, 91].