and the Quinton) and the totally implanted venous access devices that contain their own port (the Mediport, the Infuse-a-Port, and the Port-a-Cath). Several standard techniques presently are practiced for the insertion of a CVC.33 The choice of catheter and the insertion strategy depend on a number of variables, most importantly, the indication for its usage. However, patient characteristics, including any history of failed attempts, previous surgeries, comorbidities, skeletal deformities or scarring, and location of tumor, also need to be accessed.34
TABLE 41.1 Types of CVCs | ||||||
|---|---|---|---|---|---|---|
|
TABLE 41.2 Incidence of complications of CVCs | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
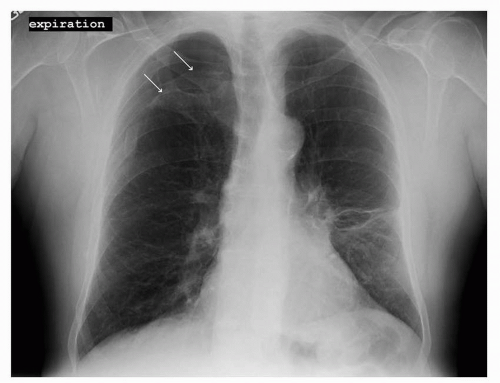 FIGURE 41-2 Pneumothorax in the right upper lobe as outlined by the two white arrows. (Courtesy of Rachel Rosovsky, MD, MPH.) |
These mural thrombi may partially or completely block the blood vessel, and their reported incidence ranges from 4% to 75%.17,25,61,62 The wide variability is due, in part, to variation in the catheter type, position, duration of insertion, and the underlying diseases. In addition, uniform standards in defining, identifying, and reporting this information are lacking.
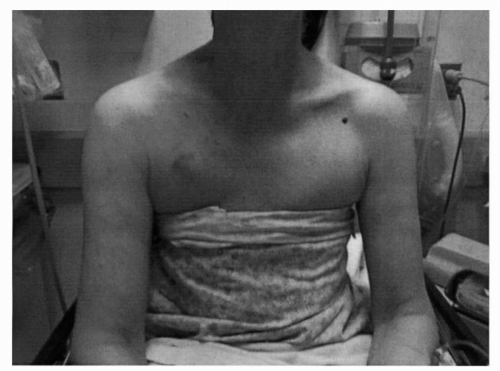 FIGURE 41-3 Venous distention and swelling of the arm due to CVC in the right SC. (Courtesy of Rachel Rosovsky, MD, MPH.) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree