FIGURE 12.1 Console of Aloka ultrasound unit. Gain knob is on bottom (long arrows); TGC slider pots are on top right (short arrows).
Echogenicity refers to the ultrasound appearance of tissues and structures. Anechoic structures appear to be without echoes; hypoechoic structures have fewer echoes, and hyperechoic structures have brighter echoes than surrounding structures. (Fig. 12.2 shows the gallbladder lumen that is anechoic or hypoechoic relative to the adjacent liver parenchyma, as well as hyperechoic stones in the gallbladder lumen.)
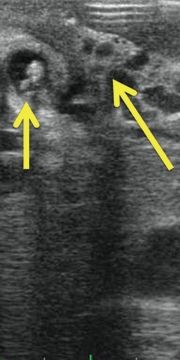
FIGURE 12.2 IOUS of the gallbladder (short arrow, note hyperechoic stones) in a patient with portal vein thrombosis and cavernous transformation (darker veins, long arrow).
Signal transmission: begins with coupling of the transducer and the structure being imaged. Coupling may be generated by direct probe placement on the tissue to be imaged (liver, pancreas, etc.) or via “standoff” through an acoustic window such as the stomach/duodenum. Flooding the operative field with saline and using this fluid as an acoustic window is a useful standoff technique with which to evaluate superficial lesions.
Probe types: Transducer frequencies ranging from 5 to 10 MHz are useful for IOUS. Many IOUS probes are set at 7.5 MHz. The sound penetration of these frequencies is 6 to 10 cm, which is acceptable for most IOUS applications. Most IOUS probes are linear array; the crystals may be arranged in a linear fashion (which generates rectangular images) or curved orientation (also called convex probes). Probes may be of the “finger” or “T” shape. Each probe shape has advantages and limitations. The operator should try as many probes as possible; most will gravitate to one probe type or another (i.e., finger or T). Laparoscopic probes may be rigid or articulate in one or two orientations; the latter are particularly helpful when scanning the posterior liver sections (Fig. 12.3).
FIGURE 12.3 Articulating laparoscopic transducers provide more range of tissue coverage.
Orientation: Two basic planes are recognized in ultrasound—longitudinal and transverse. The operator should orient the probe (particularly the T probe) by pattern recognition—the left lateral section of the liver is a useful landmark with the “point” of the left lateral section aiming to the left upper corner of the screen (Fig. 12.4). The right kidney also serves as a good landmark.
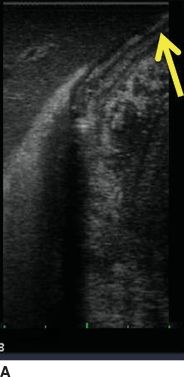
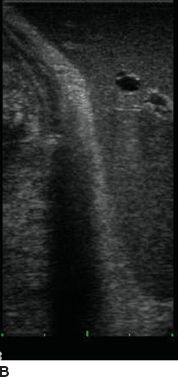
FIGURE 12.4 Orientation relative to left lateral liver section. A. Correct orientation, edge of the liver at top right of screen (arrow). B. Backward orientation.
Depth: can be adjusted in many ultrasound units (Fig. 12.1). Users should remember that image “magnification” typically sacrifices some clarity.
Image refinement: Once orientation and ideal scanning depth have been established, the image is refined by adjusting the gain and time gain compensation (TGC). Adjusting gain amplifies the returning echo signal (makes the image on the screen brighter). The gain should be set initially to make structures known to be anechoic (such as blood vessels) black. Too little gain results in nonvisualization of some structures; too much gain results in image artifact. Sound waves attenuate as they pass through tissue. The TGC controls are used to “fine tune” the depth of focus of IOUS images, using the most sensitive area of the sound wave (the focal region of transition from near field to far field) to interrogate the structure of interest.
Scanning techniques: systematic scanning is undertaken using four basic probe movements—sliding, rotating, rocking, and tilting. The novice ultrasonographer’s initial impulse is to slide the probe; however, a remarkable amount of information can be learned simply by rocking and tilting the probe from one discrete position. The operator must be aware of the effect probe pressure exerts on the underlying tissue. Too much pressure may compress vascular structures (Fig. 12.5) or obscure lesions. On the other hand, deliberate compression (“compression scanning”) is a useful technique for defining some structures. For example, in the porta hepatis, the portal vein will compress, while the hepatic artery will not compress.
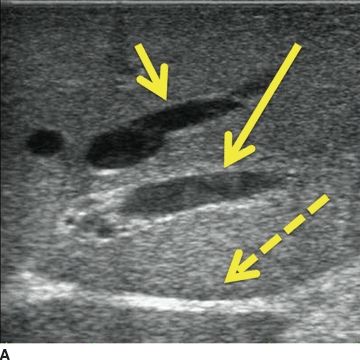
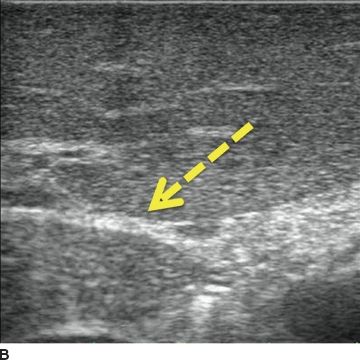
FIGURE 12.5 Compression. A. Liver without compression. Note hepatic vein (short arrow), portal vein branch (long arrow, note hyperechoic edges from extension of Glisson capsule around the portal triad), and hyperechoic ligamentum venosum (dashed arrow). B. Probe is in the same position, though with compression. Note ligamentum venosum (dashed arrow) as anatomic reference; the vascular structures have been obliterated by the compression.
Special techniques: such as standoff, acoustic window use, or intravascular contrast-enhanced ultrasound may be applied to appropriate situations.
Mode: most ultrasound images are in gray scale “B” mode (brightness modulation). Doppler mode may be used to evaluate vascular flow. In Doppler mode, spectral analysis is plotted against time. Color flow uses computer transformation of Doppler direction and velocity. By convention, flow toward the transducer is red and flow away from the transducer is blue. Velocity is typically shown in shades—darker shades indicate reduced velocity, and brighter hues representing more rapid velocity.
Annotation and storage: Images may be stored either as static images or as “cine” loop recording real-time scans.
Biopsy/ablation: biopsy or ablation may be directed precisely by ultrasound. It is critical to confirm needle position in two planes. A helpful technique is to apply color flow: moving the biopsy needle or ablation probe under color flow visualization produces color motion marking.
Table 12.1 illustrates basic approach to IOUS scanning.
TABLE 12.1 Basic Approach to IOUS Scanninga
aIOUS should be performed both prior to and after any dissection.
INTRAOPERATIVE ULTRASOUND OF THE LIVER
Intraoperative liver ultrasound is used to evaluate known lesions, identify new lesions, and guide procedures (resection, ablation, biopsy). This guidance also includes precisely localizing a lesion’s location relative to intrahepatic vascular structures, which has obvious therapeutic implications. Despite advances in cross-sectional imaging, IOUS remains the most accurate way to detect liver lesions; as many as 10% of patients will have unanticipated additional liver lesions found at the time of IOUS evaluation. This situation is particularly true for neuroendocrine tumors, many of which have numerous occult intrahepatic metastases.
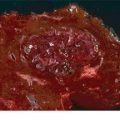
Intrahepatic metastases have a discrete acoustic character—they may be isoechoic, hyperechoic, or hypoechoic relative to the surrounding liver parenchyma (Fig. 12.6). Multiple metastases in the same patient nearly always have similar echogenicity. Therefore, the best approach to a patient with known hepatic metastases is to first identify a known lesion (i.e., one that is palpable or easily seen on cross-sectional imaging) to determine echogenicity. After identifying the echogenic characteristics of the known lesion, the entire hepatic parenchyma should be scanned systematically, keeping in mind the lesions’ character. Isoechoic lesions obviously are the most challenging to detect intraoperatively.
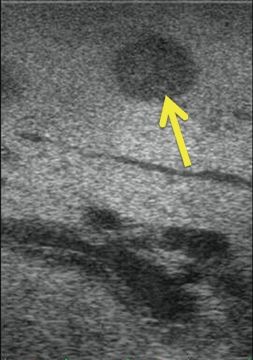
FIGURE 12.6 Metastatic cholangiocarcinoma to the liver (arrow). The lesion is hypoechoic to the surrounding hepatic parenchyma.
In general, the approach to liver IOUS involves documenting vascular inflow and outflow, followed by systematic evaluation of the parenchyma with particular attention paid to target lesions’ relationship to vascular structures. Mobilization of the liver permits more accurate ultrasonographic examination, though also creates superficial artifact. Therefore, liver IOUS should be performed both before and after mobilizing dissection.
Systematic Approach
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree