Ciliary Body Melanoma
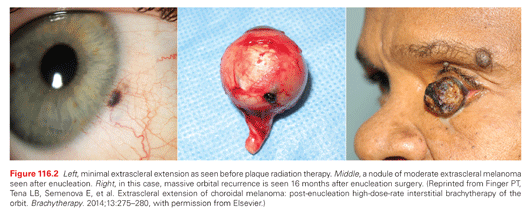
Ciliary body melanomas arise immediately posterior to the iris and are more likely to indent the adjacent lens and cause lenticular astigmatism and cataract (Fig. 116.1, middle). They can infiltrate along ciliary vessels and nerves to form nodules of epibulbar extrascleral extension (Fig. 116.2, left). The sclera above the tumor may exhibit recruited “sentinel” blood vessels. As ciliary body melanomas enlarge, they may find resistance at the iris root, leading to a circumferential growth pattern as to form an intraocular “ring” melanoma. Alternatively, they can erode through and displace the iris root into the anterior chamber.
Choroidal Melanoma
Choroidal melanomas arise posterior to the ciliary body (Fig. 116.1, right). As a choroidal melanoma grows, it typically displaces the overlying retina inwards toward the vitreous humor to form a dome shape.27 High interstitial tumor pressure, leakage, and tumor invasion combine to obliterate, erode, and weaken the overlying tissues. In 25% of cases, the choroidal melanoma breaks though its overlying Bruch’s membrane and mushrooms beneath the retina.27 A rare, alternative growth pattern is “diffuse melanoma.” This tumor is low-lying, more superficial spreading, and causes less uveal thickening.
Extrascleral Extension
Extrascleral tumor extension can occur with any size uveal melanoma (Fig. 116.2). However, it is more common with tumors located in the ciliary body, larger choroidal melanomas, or after sclera weakening by laser thermotherapy. Less common paths of extraocular and orbital extension include through the emissary vortex veins and into the optic nerve.
Finger’s “MOST” Mnemonic
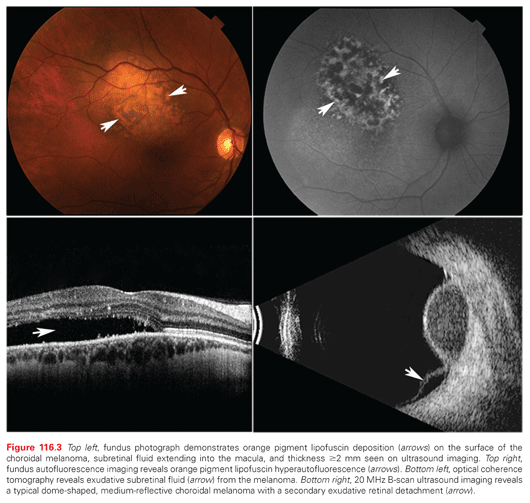
There is consensus that, when seen together, the most common characteristics of choroidal melanoma are diagnostic. As a choroidal melanoma invades and destroys the overlying retinal pigment epithelium, accumulations of orange pigment (lipofuscin and melanolipofuscin) can be visualized on the surface of a choroidal melanoma (Fig. 116.3, top left). Choroidal melanoma–associated incontinent tumor blood vessels leak serum beneath the retina, seen as overlying retinal pigment epithelial detachments or subretinal fluid. Lastly, choroidal melanomas are typically ≥2 mm thick. Therefore, Dr. Finger created the mnemonic MOST where Melanoma = Orange pigment + Subretinal fluid + Thickness of ≥2 mm. That said, not all choroidal melanomas will exhibit all three findings. However, MOST is diagnostic when all three are present.
Ophthalmic Examination
The clinical diagnosis of iris, ciliary body, and choroidal melanomas has reached a high degree of accuracy.22 In the modern era, multimodality tumor imaging can be used to clinically diagnose almost all uveal melanomas. Office-based imaging techniques range from photography, intraocular angiography, fundus autofluorescent imaging, optical coherence tomography, transillumination, and ultrasound imaging. Select cases may require orbital radiographic imaging (magnetic resonance imaging [MRI], computed tomography [CT], or positron emission tomography [PET]/CT). Biopsy is rarely required for diagnosis.22
Ophthalmic Photography and Angiography
Photographic techniques include slit-lamp, gonioscopy for iris and ciliary body tumors, as well as fundus photography for choroidal melanoma. Photography documents the current state of the affected eye, facilitates observations of tumor characteristics, and plays an important role in the diagnosis of tumor growth, posttreatment tumor response, and normal tissue side effects.
Fundus Autofluorescent Imaging
Light (500 to 750 nm, peak 630) is used to induce hyperautofluoresence from orange pigment lipofuscin.28 Thus, fundus autofluorescence can be used to detect and document one of the most important diagnostic characteristics of choroidal melanoma (Fig. 116.3, top right).
Intraocular Angiography
Angiographic photography is used to evaluate tumor microcirculation. Though some choroidal melanomas may demonstrate a pathognomonic choroidal “double circulation” pattern and/or late diffuse microaneurysms, many merely show diffuse or focal hyperfluorescence. However, these findings can be used to distinguish between the hypofluorescence of choroidal hemorrhage, the coarse vascular pattern of choroidal hemangioma, or the plethora of subretinal hyperfluorescent microaneurysms seen with angiography of choroidal metastasis.
Optical Coherence Tomography
Optical coherence tomography is a laser light-based computerized imaging system used to create tomographic slices and three-dimensional volumes of the retina, choroid, and sclera. Tumor associated subretinal and intraretinal fluid are best seen with optical coherence tomography (Fig. 116.3, bottom left).
Ophthalmic Ultrasound Imaging
Ultrasound imaging offers 0.1 mm resolution of intraocular tissues. Ultrasound imaging of choroidal melanoma typically reveals low to moderate internal tumor reflectivity, a dome shape, and/or secondary exudative retinal detachment (Fig. 116.3, bottom right).27 Less common ultrasound-imaged tumor characteristics include a mushroom shape, choroidal excavation, and extrascleral extension, as well as internal vascularity on dynamic examination. Alternative tumor-specific ultrasonographic diagnostic findings of choroidal hemangioma, osteoma, hemorrhage, or metastasis help differentiate these tumors.
High-frequency (20 to 50 MHz) ultrasonography also called ultrasound biomicroscopy (UBM) has allowed almost histopathology quality imaging of iris melanoma capable of viewing extension into the underlying pigment epithelium or adjacent ciliary body.29 Smaller ciliary body melanomas have been detected by ultrasound biomicroscopy, saving both vision and life.
Ultrasound imaging is commonly used to measure the height and basal dimensions of all uveal melanomas.27,29 Tumor measurements have been used to detect tumor growth, facilitate radiation therapy (plaque brachytherapy and proton beam), and to monitor posttreatment tumor regression.
Intraocular Tumor Biopsy
Unlike cutaneous melanoma, biopsy of uveal melanoma is controversial. With the exception of transcorneal biopsy of iris melanomas, transvitreal and transscleral biopsy requires a conjunctival and scleral incision. Discohesive tumor cells are liberated within the eye and exit through the sclerostomy into the orbit. Though no one has proved a metastatic risk associated with choroidal melanoma biopsy, known risks include vitreous hemorrhage, retinal tear, retinal detachment, infection, and cataract. Consensus indications for uveal tumor biopsy include atypical tumors, metastatic tumors, when patients require a pathology diagnosis, and for genetic/molecular analysis.30
The recent advent of genetic/molecular analysis of choroidal melanoma, its resultant prognostic information, and evolving systemic treatment protocols have prompted eye cancer specialists to recommended biopsy.31 However, the risks of biopsy must be weighed against the accuracy of clinical diagnosis and the lack of an effective treatment for metastatic disease.
UNIVERSAL STAGING AND UVEAL MELANOMA
The American Joint Committee on Cancer–International Union for Cancer Control Ophthalmic Oncology Task Force
The AJCC and The Eye Cancer Foundation sponsored The AJCC Ophthalmic Oncology Task Force with a directive to include members of the International Union for Cancer Control (UICC).32 Thus the seventh edition AJCC eye cancer staging system was created by 46 eye cancer specialists from 11 countries (France, England, Sweden, Finland, the United States of America, Canada, India, Japan, The Netherlands, Hungary, and Germany).32 These included experienced specialists in medical oncology, pediatric oncology, radiation oncology, medical physics, radiology, ocular plastic surgery, orbit, pathology, and ophthalmic oncology.
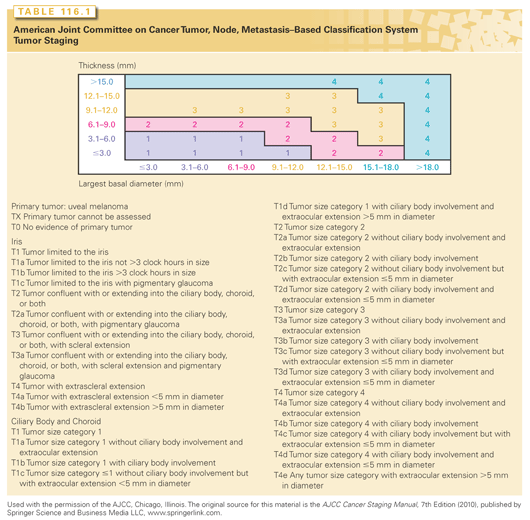
As a result, the seventh edition AJCC-UICC staging for uveal melanoma has become widely accepted.5,33–35 Most ophthalmic journals now either require or suggest authors use the seventh edition AJCC staging system in manuscript preparation. Adopted by the UICC, world cancer agencies use AJCC staging for uveal melanoma (Table 116.1). Universal staging has allowed us to better categorize and understand medical information.34,36,37 The AJCC staging system for uveal melanoma has been validated by a large single-center study and is currently being evaluated within a second large multicenter, retrospective database study (http://www.eyecancer.com/research/bioinformatics-grid-big).34,38
MANAGEMENT OF THE PRIMARY UVEAL MELANOMA
The Case for Observation of Small T1 Uveal Melanomas
Eye cancer specialists may observe small choroidal melanomas for evidence of tumor growth prior to treatment. This is because current belief is that “small choroidal melanomas” carry a low risk (≈11%) for metastases and that most current treatments risk severe vision loss. In addition, observation of small choroidal melanomas has been justified by the concept that “tumor growth demonstrates malignancy.” In practice, documented tumor growth both reassures the doctor that the tumor is malignant and reassures the patient that treatment is indicated. Specifically, the risk of treatment-related loss of vision is more than offset by a reduction in the probability of metastasis and tumor-induced vision loss.
This is particularly true for patients with small choroidal melanomas close to the fovea, monocular patients, or systemically ill patients. In these cases, serial observation may allow for years of useful vision prior to treatment. For all patients, the case for observation of small melanoma growth has been governed by the potential benefit of vision preservation (in the affected eye).
The Case Against Observation of Small T1 Uveal Melanomas
Once the eye cancer specialist is convinced that a tumor is a malignant (albeit small) choroidal melanoma, treatment becomes the most reasonable choice. In support of this approach, one can cite multiple studies that show increased tumor size (specifically largest tumor diameter) is associated with an increased risk of metastatic death.4,38,39 Therefore, it is reasonable to assume that such observations of malignant melanoma growth increase (albeit marginally) a patient’s risk for metastatic disease.
Currently, all patients with small choroidal melanoma can be offered effective eye- and vision-sparing treatments.40 They typically require particularly small radiation treatment volumes that can be associated with less vision loss. Should radiation retinopathy and optic neuropathy occur, they are treatable with intravitreal anti-VEGF medications.6–8,41
In 2013, researchers at The New York Eye Cancer Center analyzed the results of 72 small melanomas treated with palladium-103 plaque brachytherapy and followed for a mean 54 months.40 The authors noted that almost half of the eyes developed radiation maculopathy and almost 20% developed radiation optic neuropathy. However, since the advent of intravitreal anti-VEGF therapy for radiation retinopathy and optic neuropathy, only 19% or 4 of 21 affected patients lost more than two lines on the visual acuity chart. Clearly, the clinical decision “to treat or not to treat a small choroidal melanoma” is both complex and controversial (http://www.eyecancer.com/research/article/12).
Overview: Treatment of Uveal Melanoma
Fewer patients must lose their eye due to choroidal melanoma. This shift has been partly related to the COMS finding that (in treatment of select medium-sized melanomas) plaque radiation therapy was equivalent to enucleation for survival.4 This resulted in a shift toward the use of eye and vision-sparing treatments within subspecialty eye cancer centers.5
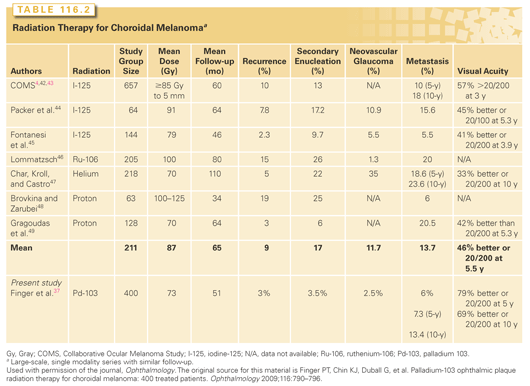
Radiation therapy is presently the most widely used eye-sparing treatment. Methods include brachytherapy plaque techniques and external beam radiation using photons, charged particles, stereotactic radiosurgery, or multisource cobalt units.5,35 The techniques with the most widely reported clinical experience are plaque brachytherapy and proton beam. A comparison of clinical outcomes is given in Table 116.2.4,37,42–49
Episcleral Plaque Radiation Therapy
Low energy plaques (iodine-125 [125I] or palladium-103 [103Pd]) are bowl-shaped devices constructed to house rice-sized radioactive sources (or seeds).35 Ruthenium-106 (106Ru) plaques are solid disc-shaped electron-emitting sources where the beta-radionuclide is covered by a 0.1 mm thick layer of silver at its episcleral aperture.35 Low-energy plaque construction and dosimetry requires a multispecialty team that integrates information about each tumor size and location from data derived from clinical examination, photography, and ultrasound imaging.35,50 The plaque is temporarily sutured to the sclera overlying the tumor, left in place for 5 to 7 days, and then surgically removed.5
Recent research suggests that dose to the fovea and lens can be correlated to the incidence of radiation maculopathy and cataract, respectively.51,52 However, each type of plaque has characteristic patterns of dose distribution within the eye. For example, 106Ru plaques emit electrons with limited intraocular penetration and less side-scatter penumbra (compared to 103Pd or 125I). Conversely, 103Pd and 125I offer greater intraocular penetration and more side scatter. All these characteristics must be taken into account when choosing a radionuclide source and plaque size.35,50 Lastly, it is important to consider dose to normal ocular structures, because side effects may be predicted based on these measurements.20,51–53
The 2014 American Brachytherapy Society (ABS) guidelines for ophthalmic plaque radiation therapy include potential methods to decrease ocular morbidity and patient mortality.5 Specifically, that ophthalmic radiation therapy should be performed in subspecialty eye cancer referral centers, that radiation oncologists and medical physicists follow the American Association of Physicists in Medicine–ABS guidelines for plaque construction, dosimetry, and quality assurance, that preoperative comparative dosimetry of available radiation sources be considered prior to treatment and that centers use the seventh edition AJCC staging system to periodically report clinical outcomes.5,33,35,50
Proton Beam
Proton beam for uveal melanoma is available at 13 institutions around the world. Pioneered at Harvard University, typical treatment involves surgical placement of metallic markers on the episclera around the tumor’s base.49 Then protons are administered over 4 consecutive days and thus four high-dose-rate treatment fractions to a prescription dose of 60 Gy. Proton therapy has been effective for local tumor control, preservation of the globe, and functional vision (see Table 116.2).
However, in contrast to low-energy plaque therapy, proton beam typically requires an anterior entrance dose resulting in more commonly reported lash loss, dry eye, neovascular glaucoma, and cataract.54 Also unlike plaques, proton treatment can be confounded by eye movement leading to a mobile target volume.54
Two clinical studies compared plaque versus proton.55,56 Both reported that charged particles offered local tumor control and reasonable vision retention compared to plaque brachytherapy. However, anterior segment complications, particularly iris neovascularization, glaucoma, and secondary enucleation, were higher after proton beam therapy.57
Iris and Iridociliary Melanoma
The overall 5-year risk for iris melanoma metastasis is 10.7%.16 However, small iris melanomas are often observed for growth prior to treatment. Surgical iridectomy or iridocyclectomy used to be the most common treatment. However, there has been a transition to relatively safe, tissue-sparing radiation therapy techniques.58–60 For example, plaque brachytherapy avoids the uncommon risks of intraocular surgery: infection, hemorrhage, acute cataract, and retinal detachment. Radiation treatment offers iris retention, retained iris function, and relatively large treatment margins. With long-term follow-up, epicorneal 103Pd plaque brachytherapy has been associated with excellent local control, a high-risk of radiation cataract formation, and almost no risk for radiation-related keratopathy, retinopathy, or optic neuropathy.53
Choroidal Melanomas that Touch or Surround the Optic Disc
Standard plaque placement covers the tumor plus a 2- to 3-mm surrounding margin.5,35 However, obtaining standard plaque placement becomes difficult when choroidal melanoma margins are near, touch, or surround the optic disc. Ophthalmoscopy typically reveals a 1.75-mm optic disc diameter, with its edge located 3.4 mm nasal to the fovea. However, just behind the eye, the optic nerve is enveloped by a 5-mm diameter optic nerve sheath.61
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








