Intracranial Hemorrhage and Subarachnoid Hemorrhage
Timolaos Rizos
Thorsten Steiner
INTRACEREBRAL HEMORRHAGE
Epidemiology, Risk Factors and Prognosis
Intracerebral hemorrhage (ICH) is an extravasation of blood into the brain parenchyma. Hemorrhagic stroke accounts for approximately 15% of all strokes.1,2 Advances in treatment (in the context of specialized neurologic and neurosurgic intensive care units) have resulted in improved survival over the last decade.3 However, the mortality rate in patients with ICHs remains high; approximately 50% of patients die in the first 3 months,4,5,6 with half of these patients dying within the first 2 days after symptom onset.4 Moreover, ICH is associated with significant morbidity. For example, after 6 months only 20% of patients are functionally independent.4
ICH is most common in elderly persons.7 The crude risk ratio for age (every 10-year increase) was calculated to be 1.97.7 The incidence of ICH is higher in African American and Asian populations compared with whites. The Northern Manhattan Study revealed that Hispanic adults from the Caribbean and Black adults have a higher risk for ICH than whites.8 Especially in elderly patients, cerebral amyloid angiopathy (CAA) is an important risk factor for primary ICH.31,32 Many modifiable risk factors for ICH have been identified and prevention of these factors may reduce the incidence of ICH. Arterial hypertension is one of the most important risk factors for ICH. In elderly patients and in patients with untreated or uncontrolled hypertension, the risk of ICH is doubled.7,17,18 The frequency of arterial hypertension in ICH has been estimated at 70% to 80%4 and the crude odds ratio (OR) for hypertension is 3.68.7 Although hypertension is the most important modifiable risk factor for prevention of ICH recurrence, data on optimal blood pressure control are lacking.19,20
The use of oral anticoagulation with warfarin is associated with an 8- to 19-fold increase in the risk of ICH.21,11 The risk of ICH in patients taking oral anticoagulants is estimated to be 0.3% to 3.7% per year (when the international normalized ratio [INR] is between 2.0 and 4.5).15 Each increase in INR of 0.5 elevates the bleeding risk by a factor of 1.4. The risk is particularly high in patients whose INR exceeds 3.9.22 Because of the rising prevalence of atrial fibrillation—which is the most frequent indication for long-term therapy with vitamin K antagonists— the burden of oral anticoagulant-associated ICH is expected to rise considerably over the next several years.14,15,16 Although more studies are needed, newer oral anticoagulants, including factor Xa inhibitors may reduce the risk for ICH when used for prevention of stroke in patients with atrial fibrillation.23,24 Highdose aspirin has also been implicated as a risk factor for ICH risk, particularly in elderly patients with untreated hypertension.25 Aspirin use (at doses that reduce the risk of myocardial infarction and ischemic stroke) is associated with an absolute risk increase in ICH of 12 events per 10,000 persons.25 In a pooled analysis from 21 cohorts, aspirin use at the time of ICH onset was independently associated with increased mortality, but not with poor functional outcome.25 The combined use of aspirin and clopidogrel further increases the risk of ICH.25 Other risk factors for ICH have been reported, including alcohol consumption,26,7 cigarette smoking, cocaine use,7 high cholesterol level,27 Vitamin E intake, and racial variations.28,29
Age over 80 years represents a risk factor for unfavorable outcome. In addition, hematoma volume and hematoma location contribute to prognosis and outcome.9,8 Specifically, large infratentorial and deeply located hematomas have unfavorable prognoses.4 ICH associated with the use of oral anticoagulants accounts for 10% to 18% of all ICH and results in particularly high rates of secondary hematoma enlargement (up to 54% of patients) and mortality (up to 67%).10,11,12,13
Pathophysiology
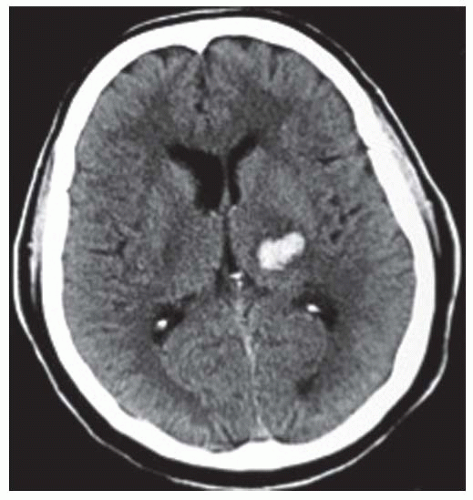
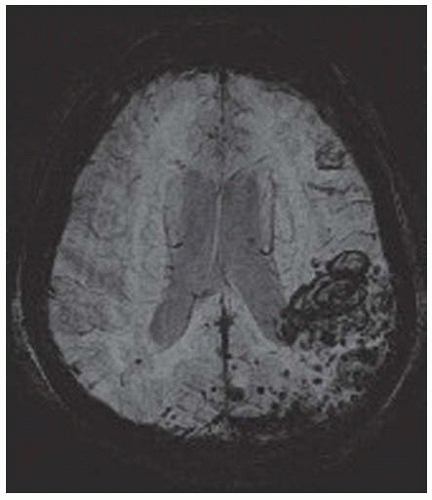
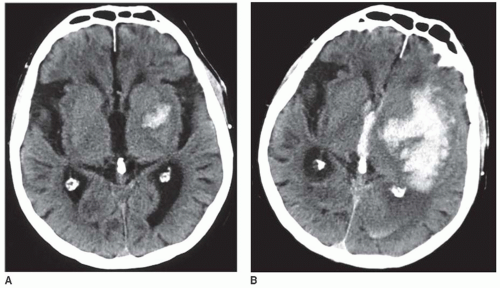
The majority of ICH (80% to 85% of all ICH) occur following the spontaneous rupture of small arteries and arterioles that are damaged by chronic arterial hypertension (up to 70% cases) or amyloid angiopathy (up to 30% cases) (FIGURES 73.1 and 73.2).17,33 These are termed “typical” or “primary” ICH. The location of the hemorrhages is usually deep, but they may also occur in the posterior fossa (brainstem and cerebellum).4 In the case of CAA, hemorrhage is frequently located in the occipital or parietal region.34 Miliary aneurysms (Charcot Buchard aneurysms) and lipohyalinosis, both associated with chronic arterial hypertension, have also been proposed as etiologic factors.
The remaining cases (15% to 20% of all ICH) are categorized as “atypical” or “secondary” ICH. These are related to multiple causes, including arteriovenous malformation (AVM), cavernoma, neoplasms, aneurysms, vasculitis, Moya-Moya disease, sinus venous thrombosis, eclampsia, and coagulopathies (e.g., liver malfunction and hemophilia). They also occur in the context of trauma or ischemic stroke, and in association with oral anticoagulation and/or antiplatelet treatment (particularly when given in combination), and drug consumption (i.e., amphetamines, cocaine).
Clinical Presentation
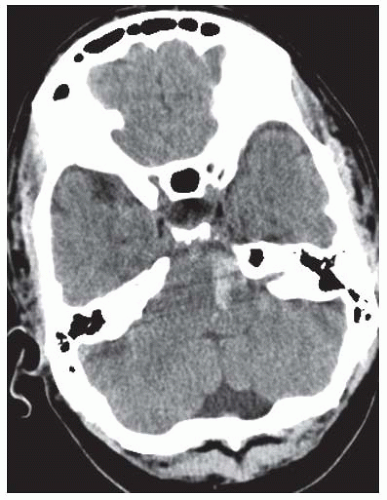
A frequent characteristic feature of ICH is the sudden onset of focal neurologic deficits during physical activity.1 Presentations of neurologic deficits depend on the bleeding location and hematoma volume. For example, a small hematoma in
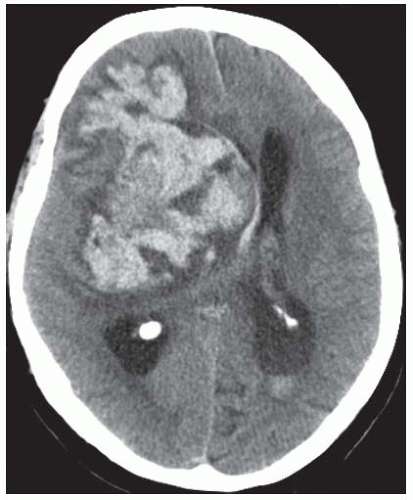
the brainstem (FIGURE 73.3) may produce profound neurologic deficits, whereas a similarly sized hematoma within supratentorial structures may cause only mild deficits. Bleeding in the basal ganglia may result in sensorimotor hemiparesis on the contralateral side. Bleeding in thalamic structures typically presents with paraesthesia or sensory loss. Symptoms of patients with lobar hematomas (˜20% of ICH) include aphasia, neglect, hemiparesis, and hemianopia. Hematomas in frontal regions may lead to neuropsychological disorders. Brainstem symptoms, including diplopia, dysarthria, dysphagia, facial weakness, and cerebellar signs such as vertigo and ataxia can be observed in infratentorially located hematomas (see FIGURE 73.3). ICH in the brainstem or supratentorial ICH with impending or apparent herniation may lead to decreased consciousness and coma. In supratentorial ICH, unequal pupils with a dilated pupil on the side of the supratentorial hematoma (resulting from pinching the third cranial nerve to the clivus bone) indicates herniation of the temporal lobe (FIGURE 73.4). Clinical deterioration is frequent in ICH, often within the first 24 hours after onset of symptoms. Underlying mechanisms include hematoma enlargement, development of perihematomal edema, hydrocephalus, and seizures. As a result, continuous monitoring of vital parameters is indicated.
the brainstem (FIGURE 73.3) may produce profound neurologic deficits, whereas a similarly sized hematoma within supratentorial structures may cause only mild deficits. Bleeding in the basal ganglia may result in sensorimotor hemiparesis on the contralateral side. Bleeding in thalamic structures typically presents with paraesthesia or sensory loss. Symptoms of patients with lobar hematomas (˜20% of ICH) include aphasia, neglect, hemiparesis, and hemianopia. Hematomas in frontal regions may lead to neuropsychological disorders. Brainstem symptoms, including diplopia, dysarthria, dysphagia, facial weakness, and cerebellar signs such as vertigo and ataxia can be observed in infratentorially located hematomas (see FIGURE 73.3). ICH in the brainstem or supratentorial ICH with impending or apparent herniation may lead to decreased consciousness and coma. In supratentorial ICH, unequal pupils with a dilated pupil on the side of the supratentorial hematoma (resulting from pinching the third cranial nerve to the clivus bone) indicates herniation of the temporal lobe (FIGURE 73.4). Clinical deterioration is frequent in ICH, often within the first 24 hours after onset of symptoms. Underlying mechanisms include hematoma enlargement, development of perihematomal edema, hydrocephalus, and seizures. As a result, continuous monitoring of vital parameters is indicated.
Diagnosis
It is important to distinguish between ICH, ischemic stroke, and subarachnoid hemorrhage (SAH). Despite efforts to establish clinical “rules” for differentiating ICH from ischemic stroke,35 definitive diagnosis requires cranial imaging (cranial computed tomography [CCT] or magnetic resonance imaging [MRI]). The sensitivity for detecting acute ICH using noncontrastenhanced CCT approaches 100%. Acute ICH presents as hyperdensity on computed tomography images (in patients with anemia, the hematomas may be isodense). During the course
of absorption, the hematoma becomes isodense, and finally hypodense. With the implementation of susceptibility-weighted T2* sequences in standard examination protocols, the sensitivity of MRI in detecting hyperacute and acute ICH is comparable to that of CCT.36 In hyperacute hemorrhage, these sequences (for a 1.5-Tesla investigation) reveal characteristic hyperintense signals. In the chronic stage, hemosiderin causes a hypointense signal on T2- and T2*-weighted sequences. Whether further imaging studies (with the exception of a 24-hour scan to detect subclinical hematoma enlargement) are needed in patients with “typical” ICH (a previous diagnosis of arterial hypertension and localization of the hematoma in the putamen, pallidum, internal capsule, pons, or cerebellum) or with suspected CAA (periventricular white matter lesions) is controversial.37 However, in younger patients without known arterial hypertension and in patients with suspected secondary bleeding (e.g., lobar bleedings or so-called “atypical” ICH) further diagnostic work-up is required to clarify the etiology of the hemorrhage. Computed tomography angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA) provide additional information in these patients. CTA provides a rapid and sensitive method for identifying vascular pathology, including aneurysms (>3 mm), and AVM. However, in patients with large ICH, hemodynamic changes may obscure detection of AVM by MRA or CTA. In these cases, DSA should be performed. MRA is the optimal technique for detecting underlying vascular malformations of low-pressure/venous systems (i.e., cavernoma and hemorrhagic tumors). Diagnosis of sinus venous thrombosis (which can cause secondary hemorrhage) requires venous MRA (contrast-enhanced) or DSA.
of absorption, the hematoma becomes isodense, and finally hypodense. With the implementation of susceptibility-weighted T2* sequences in standard examination protocols, the sensitivity of MRI in detecting hyperacute and acute ICH is comparable to that of CCT.36 In hyperacute hemorrhage, these sequences (for a 1.5-Tesla investigation) reveal characteristic hyperintense signals. In the chronic stage, hemosiderin causes a hypointense signal on T2- and T2*-weighted sequences. Whether further imaging studies (with the exception of a 24-hour scan to detect subclinical hematoma enlargement) are needed in patients with “typical” ICH (a previous diagnosis of arterial hypertension and localization of the hematoma in the putamen, pallidum, internal capsule, pons, or cerebellum) or with suspected CAA (periventricular white matter lesions) is controversial.37 However, in younger patients without known arterial hypertension and in patients with suspected secondary bleeding (e.g., lobar bleedings or so-called “atypical” ICH) further diagnostic work-up is required to clarify the etiology of the hemorrhage. Computed tomography angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA) provide additional information in these patients. CTA provides a rapid and sensitive method for identifying vascular pathology, including aneurysms (>3 mm), and AVM. However, in patients with large ICH, hemodynamic changes may obscure detection of AVM by MRA or CTA. In these cases, DSA should be performed. MRA is the optimal technique for detecting underlying vascular malformations of low-pressure/venous systems (i.e., cavernoma and hemorrhagic tumors). Diagnosis of sinus venous thrombosis (which can cause secondary hemorrhage) requires venous MRA (contrast-enhanced) or DSA.
Complications
Hematoma Enlargement
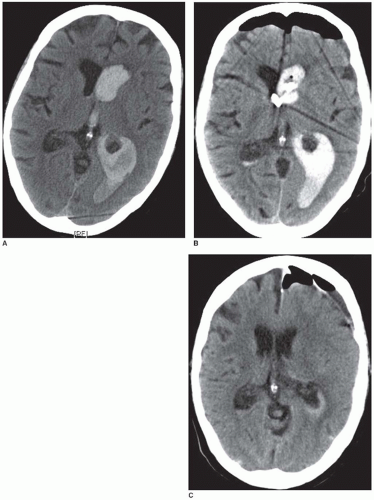
The most important complication associated with ICH is enlargement of the hematoma (FIGURE 73.5). Hematoma expansion is frequently associated with neurologic deterioration and has an unfavorable outcome. The mechanisms of hematoma expansion are not fully understood. They may involve local tissue distortion, disruption of small vessels, blood-brain barrier disruption, and local coagulopathy due to the release of tissue thromboplastin.30 ICH expansion may reflect continued bleeding or rebleeding. In approximately one-third of patients, the hematoma increases approximately 33% within the first 4 hours of onset. An additional 12% of patients suffer hematoma enlargement within the next 20 hours.38 Different predictors of hematoma expansion have been identified: large initial hematoma volume, irregular hematoma contours, heterogeneity of density on CT images, spot signs on CTA within the hematoma, liver dysfunction, hypertension, hyperglycemia, and high alcohol consumption.39,40,41,43 However, reliable radiographic predictors of ICH enlargement do not currently exist.42
Intraventricular Hemorrhage and Hydrocephalus
Concomitant bleeding into the ventricles (intraventricular hemorrhage [IVH]) is present in up to 40% of patients with ICH and portends a worse outcome.30 Furthermore, IVH is frequently associated with obstructive hydrocephalus, which often requires temporary or permanent intraventricular drainage (FIGURE 73.6). Risk factors for further enlargement of IVH include baseline mean arterial blood pressure above 120 mmHg, larger baseline ICH volume, and existence of IVH on the baseline CT scan. Predictors of death or severe disability include older age, lower baseline Glasgow coma score (GCS), larger baseline ICH volume, IVH growth >2 mL, and existent IVH at baseline or at 24 hours.44
Perihematomal Brain Edema
Perihematomal brain edema occurs is some patients with ICH. At one time, it was considered to represent ischemic penumbra (the term generally used to define ischemic but still viable cerebral tissue). However, the use of MRI has failed to confirm the existence of salvageable tissue. Indeed, perihematomal hypoperfusion has been shown to follow metabolic changes rather than true tissue ischemia.47 A more likely mechanism for perihematomal brain edema is an underlying inflammatory response to the release of thrombin and other end products of coagulation from the hematoma, and secondary disruption of the blood-brain barrier.45,46,48,49,50 Although studies are lacking, it is interesting to speculate that the use of anti-inflammatory drugs would mitigate this complication.51
Conservative Treatment
General Measures
ICH is an emergency and has to be treated as such. Although treatment options are limited, advances in supportive care have reduced mortality of these patients during the last several years. Indeed, previous studies have shown that treatment at specialized neurologic units can reduce mortality and improve functional outcome.1 In these settings, patients may be continuously monitored for vital parameters and neurologic status using
standardized scores (e.g., National Institutes of Health Stroke Scale, Scandinavian Stroke Scale, Hemphill-ICH-Score). Airway management including tracheal intubation and ventilation is frequently necessary in unconscious patients.52 Elevated body temperature (>37.5°C) and hyperglycemia should be avoided and rapidly treated. Further aspects of general treatment are the prevention of decubital ulcerations and infections. Early mobilization and rehabilitation are recommended for clinically stable patients.1
standardized scores (e.g., National Institutes of Health Stroke Scale, Scandinavian Stroke Scale, Hemphill-ICH-Score). Airway management including tracheal intubation and ventilation is frequently necessary in unconscious patients.52 Elevated body temperature (>37.5°C) and hyperglycemia should be avoided and rapidly treated. Further aspects of general treatment are the prevention of decubital ulcerations and infections. Early mobilization and rehabilitation are recommended for clinically stable patients.1
Blood Pressure Management
Arterial hypertension is one of the most important risk factors for ICH and blood pressure monitoring and management is the most important aspect of ICH treatment. In ischemic stroke, aggressive lowering of the blood pressure is associated with increased neurologic deterioration.53 However, small-volume ICH is not associated with detectable ischemic penumbra,47 and thus the risk-benefits of treating high blood pressure in this patient population may favor such intervention. Indeed, The INTEnsive blood Pressure Reduction Trial54 revealed that early intensive blood pressure-lowering treatment is clinically feasible, well tolerated, and seems to reduce hematoma growth in ICH. However, patients with severe injury (GCS 3-5) were excluded from this study, and it is still not known whether aggressive blood pressure reduction confers an improved clinical outcome. It is generally recommended that cerebral perfusion pressure (CPP) be monitored particularly in patients with elevated intracranial pressures, and CPP (mean arterial pressure minus intracranial pressure) should be kept above 60 to 80 mm Hg.
Hemostatic Therapy
Previous efforts have focused on the role of hemostatic agents in controlling bleeding and preventing hematoma expansion in ICH. Most of these strategies, including tranexamic acid, epsilon-aminocaproic acid, and aprotinin, have failed to demonstrate efficacy. However, the use of recombinant factor VIIa (rFVIIa) may hold more promise. A prospective randomized controlled phase IIB trial demonstrated the potential of rFVIIa to reduce hematoma expansion and to improve clinical outcome.55 A subsequent phase III trial (FAST-Trial) confirmed that 80 µg/kg of rFVIIa IV reduced hematoma expansion significantly when given within 4 hours. However, in contrast to the Phase IIB study, rFVIIa did not improve clinical outcome,56 and was associated with a dose-dependent increase of thromboembolic events.57 Additional studies are needed to determine the risk-benefit profile of rFVIIa treatment according to patient age, time to treatment, volume of ICH, and presence of IVH.58
Treatment of ICH Related to Oral Anticoagulants
In patients with ICH associated with the use of oral anticoagulants, normalizing coagulation is of utmost urgency. Although fresh frozen plasma may be used, it requires large volumes (which increase the risk of congestive heart failure and pulmonary edema) and is associated with substantial time delays before normalization of the INR.59,60 Thus, prothrombin complex concentrate (PCC) is preferred by many emergency physicians. PCC, a pooled plasma product containing factors II, IX, X, and (in some products) VII, can be stored in the emergency room, enabling prompt availability. However, plasma concentrations of the clotting factors can vary considerably between
different manufacturers and lots, and thus it is difficult to predict the dose needed to reverse the INR.61 Though rFVIIa can rapidly normalize INR in subjects receiving anticoagulation treatment with warfarin, its effect on bleeding duration is unclear and thus routine use of rFVIIa is currently not recommended.62,1 Treatment with coagulation factors should be combined with Vitamin K because the half-life times of coagulation factors are shorter than the half-life of warfarin. The exact time point at which the physician can safely continue oral anticoagulation after ICH in the individual patient remains unknown. It may depend on hematoma volume and location, but prospective studies with a high number of patients are needed. Given that the annual embolic risk in patients with mechanical heart valves is about 5% to 10%, discontinuation of anticoagulation for a period of 10 to 14 days seems to be an acceptable trade-off (translated 2-week risk of embolism: 0.2% to 0.4%).63
different manufacturers and lots, and thus it is difficult to predict the dose needed to reverse the INR.61 Though rFVIIa can rapidly normalize INR in subjects receiving anticoagulation treatment with warfarin, its effect on bleeding duration is unclear and thus routine use of rFVIIa is currently not recommended.62,1 Treatment with coagulation factors should be combined with Vitamin K because the half-life times of coagulation factors are shorter than the half-life of warfarin. The exact time point at which the physician can safely continue oral anticoagulation after ICH in the individual patient remains unknown. It may depend on hematoma volume and location, but prospective studies with a high number of patients are needed. Given that the annual embolic risk in patients with mechanical heart valves is about 5% to 10%, discontinuation of anticoagulation for a period of 10 to 14 days seems to be an acceptable trade-off (translated 2-week risk of embolism: 0.2% to 0.4%).63
Treatment of ICH Related to Heparin
When ICH develops in patients being treated with unfractionated heparin, rapid normalization of coagulation (i.e., the activated partial thromboplastin time) with protamine sulfate (PS) is the treatment of choice. A relevant side effect of PS is hypotension; maximum infusion rate should not exceed 5 mg PS/min and the total dose should not exceed 50 mg of PS.34
Treatment of ICH Related to Fibrinolysis
Patients with ischemic stroke who have been received thrombolytic therapy with recombinant tissue-type plasminogen activator (rt-PA) may develop ICHs. While “any” hemorrhage after stroke treatment with rt-PA is found in approximately 30% of patients, symptomatic hemorrhage (as defined by the presence of clinical deterioration attributable to hemorrhage) occurs in 3% to 9% of cases.64,65 Symptomatic hemorrhages in these patients are often serious and multifocal. Mortality at 30 days is 60% and above. To rapidly correct the systemic fibrinolytic state, platelet and factor VIII substitution is recommended.1
Management of Increased Intracranial Pressure
Increased intracranial pressure caused by cerebral edema and mass effects is frequently seen in patients with high-volume ICH and is associated with high morbidity and mortality. Because elevated intracranial pressure compromises CPP, it must be monitored for and treated in order to maintain a target CPP >60 to 70 mm Hg. Preventative measures include avoidance of situations that might increase the intracranial pressure (e.g., pain, fever, constipation, hyponatremia, and psychological/physical stress), treatment with sedation and analgesia, and maintenance of sufficient venous flow in the jugular veins (i.e., by positioning the head 30 degrees upright). Osmotic diuretics such as glycerol and mannitol (20%) can be used to lower elevated intracranial pressure. A risk of this approach, especially with mannitol, is excessive depletion of the intravascular volume, secondary renal failure, and rebound elevations in ICP. Therefore, serum levels of electrolytes, renal function, and osmolality must be carefully monitored (target osmolality: 300 to 320 mOsmol/L). Another approach is to administer hypertonic saline (Hyper-HAES: NaCl 7,5%; hydroxyethyl starch [HES] 6%). However, this treatment carries the risk of hypernatremia. In some patients, hyperventilation can rapidly reduce elevated ICP but the effect is transient and therefore this strategy is not useful for long-time therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree