Intra-Amniotic Infection
In the last 5 years, exciting new information regarding intraamniotic infection (IAI) has developed. It previously had been stated that clinically evident intrauterine infection occurred in approximately 1% of pregnancies. Prospective studies published in the last few years report rates of 4% to 10% among public and private patients. There is gathering evidence that intrauterine exposure to bacteria may be the cause of important neonatal adverse outcomes, including cerebral palsy and respiratory distress syndrome (RDS). Duration of treatment has become clearer. In addition, several strategies for the prevention of IAI have been recognized. Clinicians continue to apply several terms to this entity, including chorioamnionitis, amnionitis, intrapartum infection, amniotic fluid (AF) infection, and IAI. We use the last designation to distinguish this clinical syndrome from bacterial colonization of AF and from histologic inflammation of the cord or placenta (Fig. 17.1). However, when citing authors who use alternate expressions, we follow their terminology.
It is possible that subclinical infection of the uterine cavity and AF may lead to substantial adverse pregnancy effects, such as premature labor and premature rupture of the membranes (PROM). These developments are discussed in Chapter 18.
PATHOGENESIS
Prior to labor and rupture of the membranes (ROM), the amniotic cavity nearly always is sterile. The physical and chemical barriers formed by the intact placental membranes and cervical mucus usually are effective in preventing entry of bacteria. With the onset of labor or with ROM, bacteria from the lower genital tract commonly ascend into the amniotic cavity. In some patients, the numbers of bacteria increase with the interval after ROM. This ascending route is the most common pathway for development of IAI. A hematogenous or transplacental route of infection occurs with Listeria monocytogenes. Amniotic infection with this aerobic Gram-positive rod may result in fetal demise. Other virulent organisms, such as group A streptococci, may lead to a similar bloodborne infection.
Intra-amniotic infection has been reported in 1% to 18% of women after a cervical cerclage, especially when there is advanced dilation. Intra-amniotic infection may occur as a complication of invasive diagnostic procedures, such as amniocentesis, intrauterine transfusion, chorionic villus sampling, and percutaneous umbilical blood sampling. With diagnostic amniocentesis, amnionitis has been reported in 0% to 1% of cases. With intrauterine transfusion, infection is reported to develop in approximately 10%. Chorioamnionitis is a rare complication of chorionic villus sampling. Although IAI is very rare after percutaneous umbilical blood sampling and the fetal loss rate accompanying this procedure is only 1% to 2%, infection is responsible for a high percentage of losses and may lead to life-threatening maternal complications.
Risk factors for IAI have been identified. Coitus does not appear to be a risk factor for clinical chorioamnionitis, histologic chorioamnionitis, PROM, or preterm birth. Most cases occur in deliveries at term, and risk factors are mainly those of complicated or prolonged labor. In two large investigations using logistic regression analysis, the following risk factors were identified: low parity, prolonged duration of membrane rupture, prolonged duration of labor, larger number of vaginal examinations, and duration of internal fetal monitoring. Other data from a randomized trial of active management of labor showed that chorioamnionitis occurs less frequently when labor management features early diagnosis of abnormalities and early intervention. Even though internal fetal monitoring is associated with IAI, we believe it should be used if it enables practitioners to diagnose and treat such abnormalities more efficiently.
MICROBIOLOGY
Intra-amniotic infection is polymicrobial, involving both aerobic and anaerobic bacteria. In a controlled study of AF cultures from patients with versus those without IAI, those with IAI were more likely to have 102 CFU/mL of any isolate, any number of high-virulence isolates, and more than 102 CFU/mL of a high-virulence isolate. The isolation of low-virulence organisms, such as lactobacilli, diphtheroids, and Staphylococcus epidermidis, was similar in both the IAI and control groups. Table 17.1 shows the most common AF isolates found in more than 400 cases of IAI.
Even though group B streptococci (GBS) and Escherichia coli were isolated with modest frequency (15% and 8%, respectively), they are strongly associated with either maternal or neonatal bacteremia. When GBS was found in the AF of women with IAI, maternal or neonatal bacteremia was detected in 25% of cases. When E. coli was found, maternal or neonatal bacteremia was detected in 33%. These rates of bacteremia are significantly higher (p < .05) than the 10% rate for all organisms and the 1% rate for anaerobes. Even though Gardnerella vaginalis was isolated with high frequency, its pathogenic role has remained unclear. In a case-control study, G. vaginalis was isolated with similar frequencies in IAI and control cases (24/86 [28%] vs. 18/86 [21%], respectively; p = NS), and there was no detectable maternal antibody response to this organism.
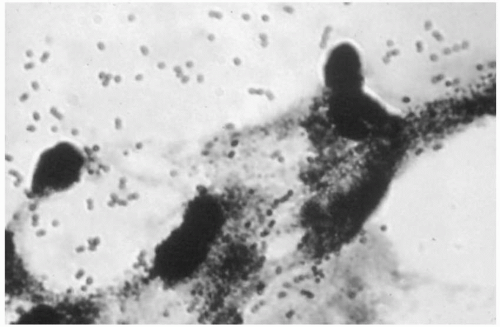
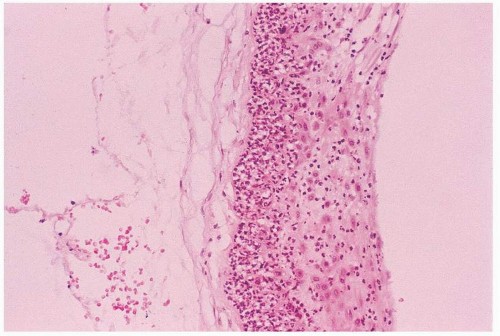 FIGURE 17.1 Histologic chorioamnionitis. Note the intense polymorphonuclear infiltrate in the membranes. |
Other microbiologic studies have reported similar isolates. On rare occasions, Neisseria gonorrhoeae may cause amnionitis. In a controlled study of IAI, 35% of AF from 52 patients with IAI yielded Mycoplasma hominis, whereas only 8% of AF from 52 matched controls had M. hominis (p < .001). Ureaplasma urealyticum was isolated from the AF from 50% of the infected and uninfected patients. Thus, M. hominis is present more commonly in the AF of infected patients but usually in association with other bacteria of known virulence. In a subsequent study, M. hominis was found in the blood of 2% of women with IAI and with M. hominis in the AF. The rate of serologic response was significantly greater than that in asymptomatic control women or infected women without IAI in the AF (p < .001). Therefore, the pathogenic potential of M. hominis is high.
Pregnant women appear to be especially susceptible to infection with L. monocytogenes, an organism that has caused regular outbreaks of infection often associated with contaminated dairy products. Maternal sepsis may occur, and fetal infection may cause demise in utero.
Several investigational approaches have revealed a strong association between bacterial vaginosis (BV) and clinical as well as histologic chorioamnionitis. First, predominant organisms involved in IAI (i.e., anaerobes and mycoplasmas plus G. vaginalis) are the same organisms found in vaginal cultures of women with BV. Second, strong associations were found among these organisms in the AF of women with IAI; when one of these organisms was found, the others were significantly more likely to be present (p < .001). Third, among at-risk women in labor, there was a significant association between a diagnosis of IAI and finding BV by Gram stain. Of women with BV, 69% (22/32) of this high-risk group had IAI, whereas of women without BV, 46% (43/93) had IAI (p = .05). Finally, there is a significant relationship between prenatal infection with BV and subsequent development of AF infection. Among 534 women in the second or third trimester, BV was present in 19%. Even though women with and without BV were similar with regard to several risk factors, AF infection occurred more often in the women with BV (9% vs. 4%; p < .05). These data suggest that antepartum treatment of BV might decrease the development of IAI, but in a randomized treatment trial of women with BV during pregnancy, the incidence of clinical amnionitis was similar in the group treated with clindamycin cream and in the group treated with placebo cream (5.9% vs. 6.3%; p = NS). Similarly, prenatal treatment of asymptomatic BV did not result in a reduction in IAI. In the several treatment trials of BV in pregnancy with oral regimens, the effect of treatment on IAI has not been reported. Bacterial vaginosis in pregnancy should be treated in symptomatic women and in women at high risk for preterm birth, such as women with previous preterm birth or preterm PROM. Currently, it is not standard practice to screen and treat all pregnant women who have BV (see also Chapter 11).
TABLE 17.1 ▪ AMNIOTIC FLUID ISOLATES IN 404 CASES OF INTRA-AMNIOTIC INFECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
HOST DEFENSE MECHANISMS
Although in most pregnant patients bacteria gain access to the amniotic cavity after ROM, few patients develop IAI. Possible defense mechanisms described are polymorphonuclear leukocytes, lysozyme, b-lysine, transferrin, immunoglobulins, and inhibitory factors. Polymorphonuclear leukocytes commonly appear in the AF of laboring women after ROM at or near term. Although their presence is associated with fever in labor, many women without symptoms have white cells in the AF, and fulminant amnionitis such as that caused by GBS may not lead to leukocytes in the AF. Among women with preterm labor with intact membranes, the AF white blood cell count (>50/mm3) had the following diagnostic indices for the detection of a positive AF culture: sensitivity, 64%; specificity, 94%; positive predictive value, 54%; and negative predictive value, 96%, in a population with a prevalence of positive cultures of 9.2% (11/120).
SEQUELAE
Once IAI develops, the fetus may swallow and aspirate the infected fluid. The infected fetus is prone to develop pneumonia, enteritis, meningitis, and sepsis because the fetal immunologic system may not be fully developed. Indeed, clinical diagnosis of chorioamnionitis is one of the most important risk factors for neonatal sepsis. One study reported an odds ratio for the development of neonatal sepsis of 25 for chorioamnionitis compared with an odds ratio of less than 5 for preterm delivery, rupture of membranes greater than 12 hours, endometritis, and GBS colonization. In the mother, endomyometritis, peritonitis, and sepsis may develop as the infection spreads outward from the amniotic cavity.
DIAGNOSIS
Diagnosis of clinical IAI requires a high index of suspicion because the clinical criteria are neither specific nor sensitive. Moreover, usual laboratory indicators of infection, such as positive stains for organisms or leukocytes and positive cultures, are found much more frequently than clinically evident infection is diagnosed.
CLINICAL CRITERIA IN THE MOTHER AND FETUS
The clinical diagnosis usually is based on fever, maternal or fetal tachycardia, uterine tenderness, foul odor of the AF, and leukocytosis. Other causes of fever in the parturient include infection of the urinary tract or other organ systems. The differential diagnosis of fetal tachycardia consists of prematurity, medications, arrhythmia, and perhaps hypoxia; other possible causes for maternal tachycardia are drugs, hypotension, dehydration, and anxiety.
In general, the most common clinical criteria are fever, leukocytosis, and ruptured membranes; fetal tachycardia and maternal tachycardia are noted in variable percentages of cases. Foul AF and uterine tenderness, although more specific signs, occur in a minority of cases. In cases of clinical chorioamnionitis, maternal fever was present in 85% to 99%, fetal tachycardia in 37% to 82%, maternal tachycardia in 19% to 37%, uterine tenderness in 13% to 16%, and foul AF in 9% to 22%.
LABORATORY CRITERIA IN THE MOTHER
To support a clinical suspicion of chorioamnionitis, the physician commonly turns to laboratory tests for assistance. A urine specimen should be obtained for analysis. A high specific gravity index may suggest dehydration, and, in a properly collected specimen, bacteriuria and pyuria suggest urinary tract infection. A portion of the urine specimen should be sent for culture and sensitivity testing. In patients with fever, bacteremia occurs in only about 10%. Because peripheral blood leukocytosis occurs commonly in normal labor, we must not rely solely on this result to suggest infection. Further, administration of corticosteroids often leads to a mild leukocytosis due to demargination of mature neutrophils.
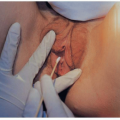
Direct examination of the AF may provide important diagnostic information (Figs. 17.2 and 17.3). We have found that samples can be collected by aspiration of an intrauterine pressure catheter in more than 50% of cases with fever in labor. Alternative routes include needle aspiration of the forewaters or amniocentesis. When the specimen is collected
by aspiration of either a catheter or the forewaters, contamination is possible. Thus, when a catheter is aspirated, the first 5 to 7 mL of fluid must be discarded. With any technique, it is helpful to perform a Gram stain and plate the specimen promptly and quantitatively to help distinguish mere contaminants from bacteria causing infection.
by aspiration of either a catheter or the forewaters, contamination is possible. Thus, when a catheter is aspirated, the first 5 to 7 mL of fluid must be discarded. With any technique, it is helpful to perform a Gram stain and plate the specimen promptly and quantitatively to help distinguish mere contaminants from bacteria causing infection.
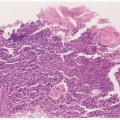
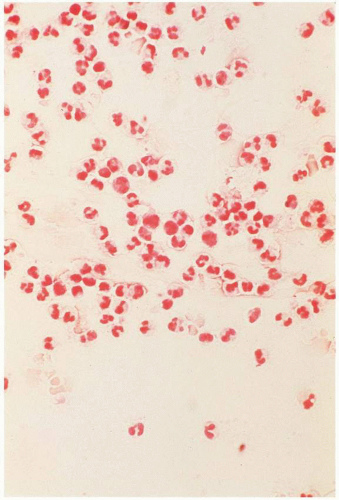 FIGURE 17.3 Gram stain of purulent amniotic fluid showing extensive numbers of polymorphonuclear leukocytes. |
In studies of patients with clinical signs of infection, investigators have found a significant association between observing bacteria in a stain of uncentrifuged AF on the one hand and colony counts greater than 102 or 103/mL and clinical infection on the other.
Determination of amniotic glucose concentration is a practical test for diagnosing clinical chorioamnionitis. A low value (<5 mg/dL) correlates with both clinical evidence of chorioamnionitis and a positive AF culture. With an amniotic glucose concentration less than 5 mg/dL, the likelihood of positive AF culture is approximately 90%. On the other hand, when the AF glucose concentration is greater than 20 mg/dL, then the likelihood of a positive culture is approximately 2%. At intermediate values, (i.e., 14 to 15 mg/dL), the likelihood of a positive amniotic culture is 30% to 50%. Amniotic fluid interleukin 6 has an even higher sensitivity and specificity but is now not available except in research settings.
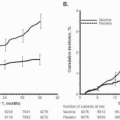
Recently, the term amniotic fluid “sludge” has been proposed to describe “free-floating hyperechogenic material within the amniotic fluid in close proximity to the uterine cervix” (Fig. 17.4). This “sludge” often resembles pus when aspirated at needle amniotomy (Fig 17.5) and may show aggregation of epithelial and white blood cells as well as bacteria on Gram stain. In one retrospective case-control study, patients with “sludge” had a significantly higher rate of spontaneous preterm delivery; a higher frequency of clinical chorioamnionitis; a higher frequency of PPROM; and a shorter median ultrasound-to-delivery interval. (See Table 17.2.) The presence of amniotic fluid
“sludge” was independently associated with microbial invasion of the amniotic cavity and histologic chorioamnionitis. The combination of short cervical length (<25 mm) and presence of “sludge” resulted in an odds ratio of 14.8 for spontaneous preterm birth less than 32 weeks.
“sludge” was independently associated with microbial invasion of the amniotic cavity and histologic chorioamnionitis. The combination of short cervical length (<25 mm) and presence of “sludge” resulted in an odds ratio of 14.8 for spontaneous preterm birth less than 32 weeks.
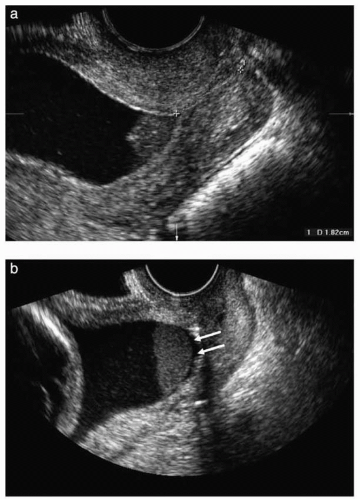 FIGURE 17.4 (A) Transvaginal ultrasound image showing the presence of dense aggregates of particulate matter (amniotic fluid “sludge”) in the proximity of the internal cervical os. (B) Similar image showing a slight detachment of the fetal membranes (arrows) at the level of the internal cervical os. The “sludge” contour is well defined, confirming that “sludge” is located in the amniotic cavity. (From Kusanovic JP, Espinoza J, Romero R. Clinical significance of the presence of amniotic fluid “sludge” in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol 2007;30:708.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|