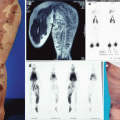
Fig. 32.1
Hemodynamic classification of AVM [2]. (a and b) Arteriovenous fistulas with one fistula between one (a) or multiple (b) artery/arteries and vein/veins. (c) Arteriovenous malformation (av angioma) with multiple communicating shunting vessels (nidus). (d) Vascular malformation (as in dural av fistula) with one or more feeding arteries and a collateral vascular network, shunting directly – fistulous type – into one draining vein
Material
Microcatheters and Microwires
Microcatheters used for the endovascular treatment of AVM are available between 1.2 and 3.0 F. Depending on the intended purpose, pushable or flow-guided microcatheters can be used. The placement of pushable microcatheters requires the use of coaxial microwires. On the other hand, flow-guided microcatheters are maneuvered passively with the use of the blood flow into the area of the AVM. The tip of the flow-guided microcatheter is floppy and, without restoring force, easily formable. Flow-guided microcatheters favor the strongest blood flow and hence can be used for atraumatic catheterization and superselective proximate embolization of the AVM nidus. Recently, new microcatheters are available that are optionally pushable and flow-guided.
Embolic Materials
Different embolic agents are used in the endovascular treatment of vascular malformations and highly vascularized tumors since the past decades [1]. Numerous methods have been developed in order to sclerotize AVM based on the endovascular injection of embolic and/or sclerosing substances. Basically, two different groups of embolic materials are employed. On the one hand, this includes so-called liquid embolic and sclerosing materials like acrylates, fibrin glues, Ethibloc®, ONYX®, strong alcohol, and Aethoxysklerol® (polydocanol). On the other hand, mechanical embolic materials can be used, for example, in the form of small particles such as polyvinyl alcohol and collagen fibers, metal spirals of platinum, tungsten or stainless steel, suture material pieces, and detachable balloons, all of which leading to a mechanical obliteration of the vessel associated with flow deceleration and subsequent thrombosis.
Liquid Embolic Materials
Acrylates like Histoacryl (n-BCA) and Glubran polymerize in contact with blood while Ethibloc®, a protein derived from maize and dissolved in alcohol, precipitates. Depending on the particular pathophysiology, Ethibloc® may be diluted with Lipiodol and, in particular, with Lipiodol plus additional alcohol [2]. Nowadays, the use of Onyx® is widely accepted in neuroradiologic interventions, and it becomes more and more important for other interventions as well [3–5]. Onyx® is a liquid embolic material consisting of ethylene vinyl alcohol copolymer dissolved in various concentrations of dimethyl sulfoxide (DMSO) and suspended micronized tantalum powder to provide contrast for fluoroscopy. The use of ethanol in the treatment of AVM will be discussed in the following chapter by another author.
Coils
Pushable coils were used in the endovascular treatment of arterial bleeding in different vascular territories since the past decades. The disadvantage of the first generation of these coils was if once deployed, retrievement was no longer possible. The next generation of coils, the electrolytic detachable coils, has been invented and introduced in the endovascular treatment of intracranial aneurysms by Guglielmi and colleagues [6]. Nowadays, we have a vast range of coils that can also be detached mechanically. Apart from the originally bare coils, we can work with hydro coils, liquid coils, fibered coils, and different types of bioactive coils. Moreover, different sizes, shapes, and forms are available from very large coils over volume coils to very small and very soft coils.
Both liquid and mechanical embolic materials have their specific fields of application. Generally speaking, liquid embolic materials may permit a vascular area to be homogeneously filled. This means that a secondary reopening of the embolized area can hardly take place. Moreover, a vascular short circuit in an area previously embolized by means of a liquid embolizate can hardly reopen by a secondary dilatation of neighboring collateral vessels. This is the reason why liquid embolizates are excellently suited for the treatment of complex plexiform AVMs. Compared to the use of particulate embolizates, they offer the advantage that the recanalization risk and frequency is significantly reduced due to the fact that the AVM is filled more completely.
However, too fast injection of liquid embolic materials may result in retrograde embolization, in the worst case of normal vessels and may furthermore lead to a glued microcatheter tip in the vessel. This risk is more often seen when using acrylates. In contrast, a passage of the embolizate into the draining vein or veins without complete occlusion of the AVM nidus may compromise physiologic venous drainage with possible complications like venous congestion with the risk of venous infarcts and bleeding. Passage of embolic material to the heart or lungs is a major feared adverse effect when dealing with uncontrollable liquid materials. Very rare but disastrous complications have been reported leading to fatal cardiovascular collapse during ethanol therapy of a venous malformation [7].
Particles like PVA are nowadays used solely for the preoperative embolization of high-vascularized tumors in order to diminish the perioperative bleeding risk and to induce a tumor necrosis. Due to their composition and size, particles do not reach and bridge the fistulous connections with their inflow zone, nidus, and outflow zone. They can occlude arterial feeders at the inflow zone only. Thus, they are not eligible in the treatment of the AVM nidus.
Indication and Safety Aspects of Embolization Procedures
As the general basis of safety aspects in any embolization procedures, mastering functional vascular anatomy and choosing the proper materials is mandatory. Specific technical complications in the interventional treatment of AVM can occur nonetheless. This might be in particular vessel perforation, undesired embolization of normal vessels that do not feed the AVM, and passage of embolic materials away from the target, for example, into the lungs or heart or into the intracranial circulation when dealing with head and neck AVM. The latter is a very feared and serious complication that may lead to stroke or hemorrhagic infarction of the brain. Therefore, highest selectivity and accuracy is performed when treating any AVM. The external carotid artery territory is linked embryological to the intracranial arteries, and thus, knowledge of functional vascular anatomy and embryology is mandatory. When treating AVM in the face or neck area, the interventionalist must know all anastomoses and supply to the cranial nerves. Usually or most of the time, extracranial-intracranial anastomoses are not visualized on serial global angiographies, but nevertheless, they do always exist. They may open under following circumstances as Geibprasert and colleagues [8] summarized: (1) with increased intra-arterial pressure, for example, during superselective injections, (2) in the presence of high-flow shunts as a consequence of the “sump effect,” or (3) as collateral routes when occlusions of the major intracranial arteries occur. These principles also apply to peripheral AVM in a similar fashion. Hence, superselective placement of the microcatheter as close as possible to the nidus or best intra-nidal, is one of the most important preventive measures to avoid embolic material passage into an undesired circulation. Another safety aspect is the continuous observation of the embolization procedure during fluoroscopy. It is important to visualize the antegrade flow of the embolic agent and to realize immediately when retrograde flow occurs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree